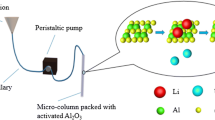
Abstract
On the basis of impregnation method, several stationary phases were prepared using γ-Al2O3 with the solution of transition metal salts and the breakthrough curves of gas chromatograph for H2 isotopes were analyzed under the temperature of liquid nitrogen. The effects of carrier gas, flow rate and doping concentration on the separation performance for H2 and D2 were systematically investigated. The overall results showed that the surface areas and adsorptive capacities of modified γ-Al2O3 were slightly lower than unmodified one while the separation performance and symmetry of chromatographic peaks of the former were more excellent. In addition, the chromatographic peaks of ortho- and para-H2 were no longer separated and the retention time shortened to half on columns of modified γ-Al2O3. All the magnetic transition metal ions modified γ-Al2O3 did very well for the separation of H2/D2 under the conditions of neon as carrier gas with a flow rate of 60 mL/min and column lengths of 1.0 m and injection amounts of 0.1 mL. Especially, the MnCl2 modified γ-Al2O3 exhibited the best performance for separating H2/D2 with an optimum doping concentration of 20 wt%.









Similar content being viewed by others
References
Beenakker, J.J.M., Borman, V.D., Krylov, S.Y.: Molecular transport in subnanometer pores: zero-point energy, reduced dimensionality and quantum sieving. Chem. Phys. Lett. 232, 379–382 (1995)
Cai, J., Pan, H., Wang, Y.: Luminescence properties of red-emitting Ca2Al2SiO7:Eu3+ nano- particles prepared by sol-gel method. Rare Met. 30, 374–380 (2011)
Cai, J., Pan, H., Wang, Y.: Synthesis and luminescence properties of Ca2SiO4-based red phosphors with Sm3+ doping for white LEDs. Int. J. Miner. Metall. Mater. 19, 663–667 (2012a)
Cai, J., Xing, Y., Zhao, X.: Quantum sieving: feasibility and challenges for the separation of hydrogen isotopes in nanoporous materials. RSC Adv. 2, 8579–8586 (2012b)
Chu, X., Zhou, Y., Zhou, L., Kou, D.: Analysis of hydrogen isotopes with gas chromatography. Chin. J. Anal. Chem. 34, 629–632 (2006)
Cunningham, C.M., Chapin, D.S., Johnston, H.L.: Separation of orthohydrogen from para- hydrogen and of paradeuterium from orthodeuterium by preferential adsorption. J. Am. Chem. Soc. 80, 2382–2384 (1958)
Cunningham, C.M., Johnston, H.L.: The surface catalysis of the ortho- to para-conversion in liquid hydrogen by paramagnetic oxides on alumina. J. Am. Chem. Soc. 80, 2377–2382 (1958)
Darkrim, F.L., Malbrunot, P., Tartaglia, G.P.: Review of hydrogen storage by adsorption in carbon nanotubes. Int. J. Hydrog. Energy 27, 193–202 (2002)
Degtyareva, O.F., Bondareva, L.T.: Gas-chromatographic analysis of mixtures of hydrogen isotopes. J. Anal. Chem. 59, 442–446 (2004)
Deng, X., Luo, D., Qin, C., Qian, X., Yang, W.: Hydrogen isotopes separation using frontal displacement chromatography with Pd–Al2O3 packed column. Int. J. Hydrog. Energy 37, 10774–10778 (2012)
Esswein, S.T., Florance, H.V., Baillie, L., Lippens, J., Barran, P.E.: A comparison of mass spectrometry based hydrogen deuterium exchange methods for probing the cyclophilin A cyclosporin complex. J. Chromatogr. A 1217, 6709–6717 (2010)
Fukada, S., Fuchinoue, K., Nishikawa, M.: Isotope separation factor and isotopic exchange rate between hydrogen and deuterium of palladium. J. Nucl. Mater. 226, 311–318 (1995)
Fukada, S., Fujiwara, H.: Comparison of chromatographic methods for hydrogen isotope separation by Pd beds. J. Chromatogr. A 898, 125–131 (2000)
Fukada, S., Samsun, B.M., Fujiwara, H.: Hydrogen absorption-desorption cycle experiment of Pd–Al2O3 pellets. Int. J. Hydrog. Energy 27, 177–181 (2002)
Grinev, T.A., Buchachenko, A.A., Krems, R.V.: Separation of ortho- and para-hydrogen in van der Waals complex formation. ChemPhysChem 8, 815–818 (2007)
Hunt, P.P., Smith, H.A.: The separation of hydrogen, deuterium and hydrogen deuteride mixtures by gas chromatography. J. Phys. Chem. 65, 87–89 (1961)
Johnson, E.R., Yang, W., Davidson, E.R.: Spin-state splittings, highest-occupied-molecular-orbital and lowest-unoccupied-molecular-orbital energies, and chemical hardness. J. Chem. Phys. 133, 164107 (2010)
Kawamura, Y., Iwai, Y., Yamanishi, T., Konishi, S., Nishi, M.: Analysis of hydrogen isotopes with a micro gas chromatograph. Fusion Eng. Des. 49–50, 855–861 (2000a)
Kawamura, Y., Konishi, S., Nishi, M.: Adsorption isotherms of hydrogen isotopes on molecular sieves 5A at low temperature. J. Nucl. Sci. Technol. 37, 536–542 (2000b)
Kawamura, Y., Konishi, S., Nishi, M.: Development of a micro gas chromatograph for the analysis of hydrogen isotope gas mixtures in the fusion fuel cycle. Fusion Eng. Des. 58–59, 389–394 (2001)
Kawamura, Y., Onishi, Y., Okuno, K., Yamanishi, T.: Hydrogen isotope separation capability of low temperature mordenite column for gas chromatograph. Fusion Eng. Des. 83, 1384–1387 (2008)
Kehimkar, B., Hoggard, J.C., Nadeau, J.S., Synovec, R.E.: Targeted mass spectral ratio analysis: a new tool for gas chromatography-mass spectrometry. Talanta 103, 267–275 (2013)
Kotoh, K., Nishikawa, T., Kashio, Y.: Multi-component adsorption characteristics of hydrogen isotopes on synthetic zeolite 5A-type at 77.4 K. J. Nucl. Sci. Technol. 39, 435–441 (2002)
Lau, J.T., Vogel, M., Langenberg, A., Hirsch, K., Rittmann, J., Zamudio-Bayer, V., Möller, T., von Issendorff, B.: Highest occupied molecular orbital-lowest unoccupied molecular orbital gaps of doped silicon clusters from core level spectroscopy. J. Chem. Phys. 134, 041102 (2011)
Li, R., Jiang, Z., Guan, Y., Yang, H., Liu, B.: Effects of metal ion on the water structure studied by the Raman O–H stretching spectrum. J. Raman Spectrosc. 40, 1200–1204 (2009)
Li, Y., Lei, X., Jockusch, S., Chen, J.Y.C., Frunzi, M., Johnson, J.A., Lawler, R.G., Murata, Y., Murata, M., Komatsu, K., Turro, N.J.: A magnetic switch for spin-catalyzed inter- conversion of nuclear spin isomers. J. Am. Chem. Soc. 132, 4042–4043 (2010)
Li, Y., Lei, X., Lawler, R.G., Murata, Y., Komatsu, K., Turro, N.J.: Distance-dependent para- H2 → ortho-H2 conversion in H2@C60 derivatives covalently linked to a nitroxide radical. J. Phys. Chem. Lett. 2, 741–744 (2011)
Liao, J., Zhang, Y., Wang, W., Xie, Y., Chang, L.: Preparation of γ-Al2O3 sorbents loaded with metal components and removal of thiophene from coking benzene. Adsorption 18, 181–187 (2012)
Moore, W.R., Ward, H.R.: The separation of orthohydrogen and parahydrogen. J. Am. Chem. Soc. 80, 2909–2910 (1958)
Moore, W.R., Ward, H.R.: Gas-solid chromatography of H2, HD, and D2 isotopic separation and heats of adsorption on alumina. J. Phys. Chem. 64, 832 (1960)
Murray, L.J., Dincǎ, M., Long, J.R.: Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009)
Naik, Y., Rao, G.A.R., Venugopal, V.: Separation of hydrogen isotopes by gas chromatogra- phy using a vanadium oxide coated molecular sieve (4A) packed column. J. Radioanal. Nucl. Chem. 247, 11–14 (2001)
Naik, Y.P., Gupta, N.K., Pillai, K.T., Rao, G.A.R., Venugopal, V.: Gas chromatographic separation of hydrogen isotopes on columns packed with alumina, modified alumina and sol-gel alumina. J. Chromatogr. A 1219, 177–179 (2012)
Nikitin, A., Li, X., Zhang, Z., Ogasawara, H., Dai, H., Nilsson, A.: Hydrogen storage in carbon nanotubes through the formation of stable C–H bonds. Nano Lett. 8, 162–167 (2008)
Peng, S., Wang, H., Fu, Y.: Tritium chemistry and techniques. Prog. Chem. 23, 1379–1385 (2011)
Samsun, B.M., Fukada, S., Fujiwara, H.: Hydrogen isotope absorption amount and rate of Pd–Al2O3 pellets. Int. J. Hydrog. Energy 26, 225–229 (2001)
Sandler, Y.L.: The adsorption and ortho-para conversion of hydrogen on diamagnetic solids. II. The relative adsorbabilities of orthohydrogen and parahydrogen. J. Phys. Chem. 58, 58–61 (1954)
Schell, J., Casas, N., Pini, R., Mazzotti, M.: Pure and binary adsorption of CO2, H2, and N2 on activated carbon. Adsorption 18, 49–65 (2012)
Schlapbach, L., Züttel, A.: Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
Stanciu, V., Stefanescu, D., David, E.: Chemically modified glasses for analysis of hydrogen isotopes by gas chromatography. J. Mater. Process. Technol. 118, 309–315 (2001)
Snyder, L.R., Kirkland, J.J., Glajch, J.L.: Practical HPLC method development, 2nd edn. John Wiley & Sons, New York (1997)
Wang, Y., Bhatia, S.K.: Quantum effect-mediated hydrogen isotope mixture separation in slit pore nanoporous materials. J. Phys. Chem. C 113, 14953–14962 (2009a)
Wang, Y., Bhatia, S.K.: Simulation of quantum separation of binary hydrogen isotope mixtures in carbon slit pores. Mol. Simul. 35, 162–171 (2009b)
Wang, L., Yang, R.T.: Hydrogen storage on carbon-based adsorbents and storage at ambient temperature by hydrogen spillover. Catal. Rev.-Sci. Eng. 52, 411–461 (2010)
Wang, X., Lu, G., Qin, C.: Preparation and characterization of gas chromatography using MnCl2\γ-Al2O3 stationary phase for on-line hydrogen isotopes analysis. Chin. J. Anal. Chem. 39, 1595–1600 (2011)
White, D., Lassettre, E.N.: Theory of ortho-para hydrogen separation by adsorption at low temperatures, isotope separation. J. Chem. Phys. 32, 72–84 (1960)
Yang, Y.X., Singh, R.K., Webley, P.A.: Hydrogen adsorption in transition metal carbon nano- structures. Adsorption 14, 265–274 (2008)
Zhao, X., Xiao, B., Fletcher, A.J., Thomas, K.M., Bradshaw, D., Rosseinsky, M.J.: Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 306, 1012–1015 (2004)
Zhao, X., Xiao, B., Fletcher, A.J., Thomas, K.M.: Hydrogen adsorption on functionalized nanoporous activated carbons. J. Phys. Chem. B 109, 8880–8888 (2005)
Zhang, D., Zhou, L., Su, W., Sun, Y.: Equilibrium modeling for hydrogen isotope separation by cryogenic adsorption. Chin. J. Chem. Eng. 14, 526–531 (2006)
Zhou, J., Gao, L., Wang, K.: Hydrogen isotope separation by cryogenic gas chromatography using the combined column of 5 Å molecular sieve and Al2O3. Int. J. Hydrog. Energy 31, 2131–2135 (2006)
Acknowledgments
We would like to thank financial supports from the “Hundred Talents Program” of Chinese Academy of Science (No. KJCX2-YW-W34) and the National Natural Science Foundation of China (No. 21073216, 21173246). We also thank Prof. Yi Wang worked at School of Applied Physics and Materials, Wuyi University, for his assistance on Raman spectra measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, J., Xing, Y., Yang, M. et al. Preparation of modified γ-alumina as stationary phase in gas–solid chromatography and its separation performance for hydrogen isotopes. Adsorption 19, 919–927 (2013). https://doi.org/10.1007/s10450-013-9499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9499-2