Abstract
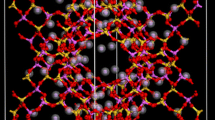
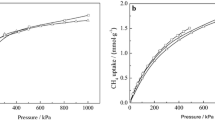
The adsorption properties of CO2, N2 and CH4 in all-silica zeolites were studied using molecular simulations. Adsorption isotherms for single components in MFI were both measured and computed showing good agreement. In addition simulations in other all silica structures were performed for a wide range of pressures and temperatures and for single components as well as binary and ternary mixtures with varying bulk compositions. The adsorption selectivity was analyzed for mixtures with bulk composition of 50:50 CO2/CH4, 50:50 CO2/N2, 10:90 CO2/N2 and 5:90:5 CO2/N2/CH4 in MFI, MOR, ISV, ITE, CHA and DDR showing high selectivity of adsorption of CO2 over N2 and CH4 that varies with the type of crystal and with the mixture bulk composition.
Similar content being viewed by others
Abbreviations
- p:
-
Pressure, Pa
- po:
-
Saturation pressure, Pa
- qSi :
-
Point charge of silicon
- qO :
-
Point charge of oxygen
References
Auerbach, S.M., Kärger, J., Vasenkov, S.: Diffusion in zeolites. In: Auerbach, S.M., Carrado, K.A., Dutta, P.K. (eds.) Handbook of Zeolite Science and Technology. Dekker, New York (2003)
Babarao, R., Hu, Z.Q., Jiang, J.W., Chempath, S., Sandler, S.I.: Storage and separation of CO2 and CH4 in silicalite, C-168 schwarzite, and IRMOF-1: a comparative study from Monte Carlo simulation. Langmuir 23, 659–666 (2007)
Baerlocher, C., Meier, W.M., Olson, D.H.: Atlas of Zeolite Structure Types, 5th edn. Elsevier, London (2001)
Bernal, M.P., Coronas, J., Menendez, M., Santamaria, J.: Separation of CO2/N-2 mixtures using MFI-type zeolite membranes. AIChE J. 50, 127–135 (2004)
Bezus, A.G., Kiselev, A.V., Lopatkin, A.A., Du, P.Q.: Molecular statistical calculation of thermodynamic adsorption characteristics of zeolites using atom-atom approximation. 1. Adsorption of methane by zeolite Nax. J. Chem. Soc. Faraday Trans. II 74, 367–379 (1978)
Bodoardo, S., Borello, L., Fiorilli, S., Garrone, E., Onida, B., Arean, C.O., Penazzi, N., Palomino, G.T.: Methylene blue encapsulated in silica-based mesophases: characterisation and electrochemical activity. Microporous Mesoporous Mater. 79, 275–281 (2005)
Breck, D.W.: Zeolite Molecular Sieves. Wiley, New York (1974)
Calero, S., Dubbeldam, D., Krishna, R., Smit, B., Vlugt, T.J.H., Denayer, J.F.M., Martens, J.A., Maesen, T.L.M.: Understanding the role of sodium during adsorption: A force field for alkanes in sodium-exchanged faujasites. J. Am. Chem. Soc. 126, 11377–11386 (2004)
Choudhary, V.R., Mayadevi, S.: Adsorption of methane, ethane, ethylene, and carbon dioxide on silicalite-I. Zeolites 17, 501–507 (1996)
Delgado, J.A., Uguina, M.A., Gomez, J.M., Ortega, L.: Adsorption equilibrium of carbon dioxide, methane and nitrogen onto Na- and H-mordenite at high pressures. Sep. Purif. Technol. 48, 223–228 (2006)
Dubbeldam, D., Calero, S., Maesen, T.L.M., Smit, B.: Incommensurate diffusion in confined systems. Phys. Rev. Lett. 90, 245901 (2003)
Dubbeldam, D., Calero, S., Vlugt, T.J.H., Krishna, R., Maesen, T.L.M., Beerdsen, E., Smit, B.: Force field parametrization through fitting on inflection points in isotherms. Phys. Rev. Lett. 93 (2004a)
Dubbeldam, D., Calero, S., Vlugt, T.J.H., Krishna, R., Maesen, T.L.M., Smit, B.: United atom force field for alkanes in nanoporous materials. J. Phys. Chem. B 108, 12301–12313 (2004b)
Dubbeldam, D., Beerdsen, E., Calero, S., Smit, B.: Molecular path control in zeolite membranes. Proc. Nat. Acad. Sci. USA 102, 12317–12320 (2005)
Frenkel, D., Smit, B.: Understanding Molecular Simulations: From Algorithms to Applications, 2nd edn. Academic, San Diego (2002)
Garcia-Perez, E., Dubbeldam, D., Liu, B., Smit, B., Calero, S.: A computational method to characterize framework aluminum in aluminosilicates. Angew. Chem. Int. Ed. 46, 276–278 (2007)
Gardner, T.Q., Falconer, J.L., Noble, R.D.: Adsorption and diffusion properties of zeolite membranes by transient permeation. Desalination 149, 435–440 (2002)
Goj, A., Sholl, D.S., Akten, E.D., Kohen, D.: Atomistic simulations of CO2 and N-2 adsorption in silica zeolites: The impact of pore size and shape. J. Phys. Chem. B 106, 8367–8375 (2002)
Grey, T.J., Travis, K.P., Gale, J.D., Nicholson, D.: A comparative simulation study of the adsorption of nitrogen and methane in siliceous heulandite and chabazite. Microporous Mesoporous Mater. 48, 203–209 (2001)
Harlick, P.J.E., Tezel, F.H.: Adsorption of carbon dioxide, methane, and nitrogen. Sep. Sc. Technol. 37, 33–60 (2002)
Harlick, P.J.E., Tezel, F.H.: Adsorption of carbon dioxide, methane and nitrogen: pure and binary mixture adsorption for ZSM-5 with SiO2/Al2O3 ratio of 280. Sep. Purif. Technol. 33, 199–210 (2003)
Harris, J.G., Yung, K.H.: Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J. Phys. Chem. 99(31), 12021–12024 (1995)
Hasegawa, Y., Kusakabe, K., Morooka, S.: Effect of temperature on the gas permeation properties of NaY-type zeolite formed on the inner surface of a porous support tube. Chem. Eng. Sci. 56, 4273–4281 (2001)
Himeno, S., Tomita, T., Suzuki, K., Yoshida, S.: Characterization and selectivity for methane and carbon dioxide adsorption on the all-silica DD3R zeolite. Microporous Mesoporous Mater. 98, 62–69 (2007)
Hirotani, A., Mizukami, K., Miura, R., Takaba, H., Miya, T., Fahmi, A., Stirling, A., Kubo, M., Miyamoto, A.: Grand canonical Monte Carlo simulation of the adsorption of CO2 on silicalite and NaZSM-5. Appl. Surf. Sci. 120, 81–84 (1997)
Jaramillo, E., Auerbach, S.M.: New force field for Na cations in faujasite-type zeolites. J. Phys. Chem. B 103, 9589–9594 (1999)
Jia, W., Murad, S.: Molecular dynamics simulations of gas separations using faujasite-type zeolite membranes. J. Chem. Phys. 120, 4877–4885 (2004)
Kiselev, A.V., Lopatkin, A.A., Shulga, A.A.: Molecular statistical calculation of gas-adsorption by silicalite. Zeolites 5, 261–267 (1985)
Krishna, R., Smit, B., Calero, S.: Entropy effects during sorption of alkanes in zeolites. Chem. Soc. Rev. 31, 185–194 (2002)
Kusakabe, K., Kuroda, T., Uchino, K., Hasegawa, Y., Morooka, S.: Gas permeation properties of ion-exchanged faujasite-type zeolite membranes. AIChE J. 45, 1220–1226 (1999)
Li, S.G., Falconer, J.L., Noble, R.D.: SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 241, 121–135 (2004)
Li, S.G., Alvarado, G., Noble, R.D., Falconer, J.L.: Effects of impurities on CO2/CH4 separations through SAPO-34 membranes. J. Membrane Sci. 251, 59–66 (2005)
Lillerud, H.R.a.K.P.: Verified Synthesis of Zeolitic Materials. Elsevier, Amsterdam (2001)
Llewellyn, P.L., Coulomb, J.P., Grillet, Y., Patarin, J., Andre, G., Rouquerol, J.: Adsorption by Mfi-type zeolites examined by isothermal microcalorimetry and neutron-diffraction. 2. Nitrogen and carbon-monoxide. Langmuir 9, 1852–1856 (1993)
Maesen, T.L.M., Beerdsen, E., Calero, S., Dubbeldam, D., Smit, B.: Understanding cage effects in the n-alkane conversion on zeolites. J. Catal. 237, 278–290 (2006)
Makrodimitris, K., Papadopoulos, G.K., Theodorou, D.N.: Prediction of permeation properties of CO2 and N-2 through silicalite via molecular simulations. J. Phys. Chem. B 105, 777–788 (2001)
Murthy, C.S., Singer, K., Klein, M.L., McDonald, I.R.: Pairwise Additive Effective Potentials for Nitrogen. Mol. Phys. 41, 1387–1399 (1980)
Nam, G.M., Jeong, B.M., Kang, S.H., Lee, B.K., Choi, D.K.: Equilibrium isotherms of CH4, C2H6, C2H4, N-2, and N-2 on zeolite 5A using a static volumetric method. J. Chem. Eng. Data 50, 72–76 (2005)
Parra, J.B., Ania, C.O., Arenillas, A., Rubiera, F., Pis, J.J., Palacios, J.M.: Structural changes in polyethylene terephthalate (PET) waste materials caused by pyrolysis and CO2 activation. Adsorpt. Sci. Technol. 24, 439–449 (2006)
Poshusta, J.C., Tuan, V.A., Falconer, J.L., Noble, R.D.: Synthesis and permeation properties of SAPO-34 tubular membranes. Ind. Eng. Chem. Res. 37, 3924–3929 (1998)
Poshusta, J.C., Noble, R.D., Falconer, J.L.: Temperature and pressure effects on CO2 and CH4 permeation through MFI zeolite membranes. J. Membr. Sci. 160, 115–125 (1999)
Ryckaert, J.P., Bellemans, A.: Molecular-Dynamics of Liquid Alkanes. Faraday Discuss. 95–106 (1978)
Schenk, M., Calero, S., Maesen, T.L.M., van Benthem, L.L., Verbeek, M.G., Smit, B.: Understanding zeolite catalysis: Inverse shape selectivity revised. Angew. Chem. Int. Ed. 41, 2500 (2002)
Sun, M.S., Shah, D.B., Xu, H.H., Talu, O.: Adsorption equilibria of C-1 to C-4 alkanes, CO2, and SF6 on silicalite. J. Phys. Chem. B 102, 1466–1473 (1998)
Tomita, T., Nakayama, K., Sakai, H.: Gas separation characteristics of DDR type zeolite membrane. Microporous Mesoporous Mater. 68, 71–75 (2004)
van den Broeke, L.J.P., Bakker, W.J.W., Kapteijn, F., Moulijn, J.A.: Transport and separation properties of a silicalite-1 membrane. I. Operating conditions. Chem. Eng. Sci. 54, 245–258 (1999)
Watanabe, K., Austin, N., Stapleton, M.R.: Investigation of the air separation properties of zeolites type-a, type-X and type-Y by Monte-Carlo simulations. Mol. Simul. 15, 197 (1995)
Webster, C.E., Cottone, A., Drago, R.S.: Multiple equilibrium analysis description of adsorption on Na-mordenite and H-mordenite. J. Am. Chem. Soc. 121, 12127–12139 (1999)
Yu, J.B., Jiang, Z., Zhu, L., Hao, Z.P., Xu, Z.P.: Adsorption/desorption studies of NOx on well-mixed oxides derived from Co-Mg/Al hydrotalcite-like compounds. J. Phys. Chem. B 110, 4291–4300 (2006)
Zhu, W.D., Hrabanek, P., Gora, L., Kapteijn, F., Moulijn, J.A.: Role of adsorption in the permeation of CH4 and CO2 through a silicalite-1 membrane. Ind. Eng. Chem. Res. 45, 767–776 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Pérez, E., Parra, J.B., Ania, C.O. et al. A computational study of CO2, N2, and CH4 adsorption in zeolites. Adsorption 13, 469–476 (2007). https://doi.org/10.1007/s10450-007-9039-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-007-9039-z