Abstract
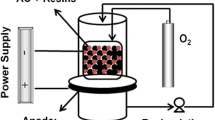
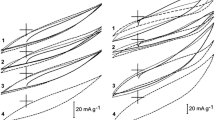
This paper investigates the use of electrochemical techniques for the removal of a common herbicide, bentazone, from water streams using a carbon-based electrode. Activated carbon cloth with high surface area and narrow micropores was used as electrode. For a better understanding of the process, adsorption was investigated under both open circuit and controlled polarization conditions, the latter in anodic and cathodic directions. It was found that anodic polarization enhances the kinetics of adsorption of the herbicide on the carbon cloth, the extent of which is strongly related to the applied current value. At converse, cathodic polarization induces the reversible desorption of the compound. Moreover, in-situ UV spectra recording on the solution did not show any structural change of the herbicide upon polarization, demonstrating the reversibility of the process for the regeneration of the adsorbent and the recovery of the compound. Based on these experiments, a mechanism is proposed to interpret the reversible sorption of bentazone under polarization.
Similar content being viewed by others
Abbreviations
- C:
-
solution concentration (mg L−1)
- Co :
-
initial solution concentration (mg L−1)
- DFT:
-
density functional theory
- DR:
-
Dubinin-Radushkevich method
- E:
-
equilibrium reduction potential (Volts)
- I:
-
applied current (mA)
- K ads :
-
adsorption rate constant (min−1)
- NHE:
-
Normal Hydrogen Electrode
- p:
-
pressure
- p0 :
-
saturation pressure
- pHPZC :
-
pH of the point of zero charge (pH units)
- PSD:
-
pore size distribution
- q:
-
amount of bentazone adsorbed (mg g−1)
- qe :
-
amount of bentazone adsorbed at equilibrium (mg g−1)
- S BET :
-
apparent specific surface area obtained from the nitrogen adsorption data at −196°C by the BET model (m2 g−1)
- t:
-
time (min)
- U:
-
working electrode potential (Volts)
- VCO2 :
-
narrow micropores volume, obtained from the CO2 adsorption data at 0°C, by the DR method (cm3 g−1)
- Vmeso :
-
mesopores volume, obtained from the nitrogen adsorption data at −196°C, by the DFT method (cm3 g−1)
- Vmicro :
-
micropores volume, obtained from the nitrogen adsorption data at −196°C, by the DFT method (cm3 g−1)
- VTOTAL :
-
total pore volume obtained from the nitrogen adsorption data at −196°C, and evaluated at p/p0∼0.95 (cm3 g−1)
References
Afkhami, A., Conway, B.E.: Investigation of removal of Cr(VI), Mo(VI), W(VI), V(IV), and V(V) oxy-ions from industrial waste-waters by adsorption and electrosorption at high-area carbon cloth. J. Colloid Interface Sci. 251, 248–255 (2002)
Alfarra, A., Frackowiak, E., Béguin, F.: Mechanism of lithium electrosorpion by activated carbons. Electrochim. Acta 47, 545–1553 (2002)
Ania, C.O., Béguin, F.: Mechanism of adsorption and electrosorption of bentazone on activated carbon cloth in aqueous solutions. Water Res. 41, 3372–3380 (2007)
Ayranci, E., Conway, B.E.: Removal of phenol, phenoxide and chlorophenols from waste-waters by adsorption and electrosorption at high-area carbon felt electrodes. J. Electroanal. Chem. 513, 100–110 (2001)
Ayranci, E., Hoda, N.: Adsorption of bentazone and propanil from aqueous solutions at the high area activated carbon cloth. Chemosphere 57, 755–762 (2004)
Bandosz, T.J.: Activated carbon surfaces for environmental remediation. In: Interface Science and Technology. Elsevier, New York (2006)
Boivin, A., Cherrier, R., Schiavon, M.: A comparison of five pesticides adsorption and desorption processes in thirteen contrasting field soils. Chemosphere 61, 668–676 (2005)
Brown, N.W., Roberts, E.P.L., Garforth, A.A., Dryfe, R.A.W.: Electrochemical regeneration of a carbon-based adsorbent loaded with crystal violet dye. Electrochim. Acta 49, 3269–3281 (2004)
Damaskin, B.B., Petri, O.A., Batrakov, V.V.: Adsorption of Organic Compounds at Electrodes. Plenum, New York (1971)
Directive 2000/60/EC. Official Journal of the European Union, L 327, on 22 December 2000
Dousset, S., Babut, M., Andreux, F., Schiavon, M.: Alachlor and bentazone losses from subsurface drainage of two soils. J. Environ. Qual. 33, 294–301 (2004)
EPA, Pesticides Industry Sales and Usage Report. United States Environmental Protection Agency Office of Prevention, Pesticides, and Toxic Substances (7503C), 733-R-04-001 (2004)
European Communities. Panorama of the European Union. Measuring progress towards a more sustainable Europe. ISBN 92-894-9768-8. Office for Official Publications of the European Communities, Luxembourg (2005)
Han, Y., Quan, X., Chen, S., Zhao, H., Cui, C., Zhao, Y.: Electrochemically enhanced adsorption of aniline on activated carbon fibers. Sep. Purif. Technol. 50, 365–372 (2006a)
Han, Y., Quan, X., Chen, S., Zhao, H., Cui, C., Zhao, Y.: Electrochemically enhanced adsorption of phenol on activated carbon fibers in basic aqueous solution. J. Colloid Interface Sci. 299, 766–771 (2006b)
Jurewicz, J., Frackowiak, E., Béguin, F.: Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A78, 981–987 (2004)
Kinoshita, K.: Carbon: Electrochemical and Physicochemical Properties, pp. 293. Wiley, New York (1988)
Niu, J., Conway, B.E.: States of orientation of pyridine and 1,4-pyrazine as a function of electrode potential and surface charge at a high-area, porous C-electrode. J. Electroanal. Chem. 564, 53–63 (2004)
Niu, J., Conway, B.E.: Development of techniques for purification of waste waters: removal of pyridine from aqueous solution by adsorption at high-area C-cloth electrodes using in situ optical spectrometry. J. Electroanal. Chem. 521, 16–28 (2002a)
Niu, J., Conway, B.E.: Molecular structure factors in adsorptive removal of pyridinium cations, 1,4-pyrazine and 1-quinoline at high-area C-cloth electrodes for waste-water remediation. J. Electroanal. Chem. 529, 84–96 (2002b)
Niu, J., Conway, B.E.: Adsorptive and electrosorptive removal of aniline and bipyridyls from waste-waters. J. Electroanal. Chem. 536, 83–92 (2002c)
Niu, J., Conway, B.E.: Adsorption of organics onto an high-area C-cloth electrode from organic solvents and organic solvent/water mixtures. J. Electroanal. Chem. 546, 59–72 (2003)
Noh, J.S., Schwarz, J.A.: Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci. 130, 157–164 (1989)
Coughlin, R.W., Ezra, F.S.: Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 2, 291–297 (1968)
Ying, T.Y., Yang, K.L., Yiacoumi, S., Tsouris, C.: Electrosorption of ions from aqueous solutions by nanostructured carbon aerogel. J. Colloid Interface Sci. 250, 18–27 (2002)
Zhou, M.H., Lei, L.C.: Electrochemical regeneration of activated carbon loaded with p-nitrophenol in a fluidized electrochemical reactor. Electrochim. Acta 51, 4489–4496 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ania, C.O., Béguin, F. Electrochemically assisted adsorption/desorption of bentazone on activated carbon cloth. Adsorption 13, 579–586 (2007). https://doi.org/10.1007/s10450-007-9024-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-007-9024-6