Abstract
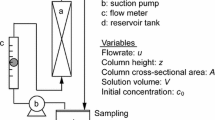
An analytical mathematical model is used to investigate the influence of minute adsorbent temperature changes on the kinetics of sorption of a gaseous adsorbate from mixture with a carrier gas using a differential closed loop recycle method. Isothermal operation may not be achieved even when a very high gas circulation rate is used. Very small changes in the adsorbent temperature during the process can cause substantial departure from isothermal uptake behavior. It is shown that the kinetic process can be assumed to be isothermal only for trace adsorbate concentrations. A criterion for validity of isothermal data analysis is proposed.
Similar content being viewed by others
References
Golden, T.C. and S. Sircar, “Equilibrium and Kinetics of Adsorption of Freon-12 at Infinite Dilution,” AIChE J., 40, 935–943 (1994).
Haul, R. and H. Stremming, “Non-Isothermal Sorption Kinetics in Porous Adsorbents,” J. Colloid and Interface Sci., 97, 348 (1984).
Leva, M, Chem. Eng., 56, 115 (1949).
Ruthven, D.M., Principles of Adsorption and Adsorption Processes, Chapter 6, John Wiley: New York (1984).
Ruthven, D.M. and K.F. Loughlin, “Sorption of Light Paraffins on Type A Zeolites: Analysis and Interpretation of Equilibrium Isotherms,” J. Chem. Soc. Faraday Trans. I, 68, 696–708 (1972).
Sircar, S, “On the Measurement of Sorption Kinetics by Differential Test: Effect of the Heat of Sorption,” Carbon, 19, 285–288 (1981).
Sircar, S., “Linear Driving Force Model for Non-Isothermal Gas Adsorption Kinetics,” J. Chem. Soc. Faraday Trans. I, 79, 785–796 (1983).
Sircar, S., “Non-Isothermal Differential Adsorption Kinetics for Binary Gas Mixtures,” Ind. Eng. Chem. Res., 33, 1585–1592 (1994).
Sircar, S. and J.R. Hufton, “Why Does Linear Driving Force Model for Adsorption Kinetics Work?,” Adsorption, 6, 137–147 (2000).
Sircar, S. and R. Kumar, “Adsorption of a Dilute Adsorbate: Effects of Small Changes in the Column temperature,” Ind. Eng. Chem. Process Des. Dev., 22, 280–287 (1983).
Sircar, S. and R. Kumar, “Non-Isothermal Surface Barrier Model for Gas Sorption Kinetics on Porous Adsorbents,” J. Chem. Soc. Faraday Trans. I, 80, 2489–2507 (1984).
Sun, L.M. and F. Meunier, “On the Heat Effect in Measurements of Sorption Kinetics by the Frequency Response Method,” Chem. Eng. Sci., 45, 715–722 (1993).
Sun, L.M., F. Meunier, and Ph. Grenier, “Frequency Response for Non-Isothermal Adsorption in Biporous Pellets,” Chem. Eng. Sci., 49, 373–381 (1994).
Vargaftik, N.B., Handbook of Physical Properties of Liquids and Gases: Pure Substances and Mixtures, Hemisphere Publishing Co.: New York (1975).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sircar, S. Gas sorption kinetics by differential closed-loop recycle method: Effect of heat of adsorption. Adsorption 12, 259–266 (2006). https://doi.org/10.1007/s10450-006-0502-z
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-006-0502-z