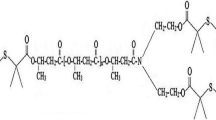
Abstract
A new polymer containing double amidoxime groups per repeating unit was synthesized to enhance the metal ion uptake capacity. The adsorption properties of this new polymeric adsorbent, amidoximated poly(N,N′-dipropionitrile acrylamide), for U(VI), V(V), Cu(II), Co(II) and Ni(II) ions were investigated by batch and flow-through processes at very low concentration levels (ppb). The chelating polymer showed high adsorption capacity for uranyl as well as vanadyl ions. In selectivity studies from a mixture of metal ions in aqueous solutions, the adsorbent showed high selectivity for uranyl and vanadyl ions in the following order: U(VI) > V(V) ≫ Co(II) = Cu(II) ≫ Ni(II) as determined by calculating the distribution coefficients D, of corresponding ions. The adsorption of uranyl and vanadyl ions from natural seawater by the new adsorbent was also examined in flow through mode.
Similar content being viewed by others
References
Akkaş, P. and O. Güven, “Enhancement of Uranyl Ion Uptake by Prestructuring of Acrylamide-Maleic Acid Hydrogels,” J. Appl. Polym. Sci., 78, 284–289 (2000).
Choi, S.H. and Y.C. Nho, “Adsorption of UO2 2 + by Polyethylene Adsorbents with Amidoxime, Carboxyl, and Amidoxime/Carboxyl Group,” Rad. Phys. Chem., 57, 187–193 (2000).
Colella, M.B., S. Sigga, and R.M. Barnes, “Synthesis and Characterization of a Poly(acrylamidoxime) Metal Chelating Resin,” Anal. Chem., 52, 967–972 (1980).
Egawa, H. and H. Harada, “Recovery of Uranium from Seawater by Using Chelating Resins Containing Amidoxime Groups,” Nippon Kagaku Kaishi, 958–959 (1979).
Egawa, H., H. Harada, and T. Shuto, “Recovery of Uranium from Seawater by the Use of Chelating Resins Containing Amidoxime Groups,” Nippon Kagaku Kaishi, 1773–1776 (1980a).
Egawa, H., H. Harada, and T. Nonaka, “Preparation of Adsorption Resins for Uranium in Seawater,” Nippon Kagaku Kaishi, 1767–1772 (1980b).
Garg, B.S., R.K. Sharma, N. Bhojak, and S. Mittal, “Chelating Resins and their Applications in the Analysis of Trace Metal Ions,” Microchemical Journal, 61, 94–114 (1999).
Güven, O., M. şen, E. Karadaĝ and D. Saraydín, “A Review on the Radiation Synthesis of Copolymeric Hydrogels for Adsorption and Separation Purposes,” Radiat. Phys. Chem., 56, 381–386 (1999).
Kantipuly, C., S. Katragadda, A. Chow, and H.D. Gesser, “Chelating Polymers and Related Supports for Separation and Preconcentration of Trace Metals,” Talanta, 37, 491–517 (1990).
Kavaklí Akkaş, P., C. Uzun, and O. Güven, “Synthesis, Characterization and Amidoximation of a Novel Polymer: Poly(N,N′-dipropionitrile acrylamide),” React. Funct. Polym., 61, 245–254 (2004a).
Kavaklí Akkaş, P., N. Seko, M. Tamada, and O. Güven, “Adsorption Efficiency of a New Adsorbent Towards Uranium and Vanadium Ions at Low Concentrations,” Sep. Sci. Technol., 39, 1631–1643 (2004b).
Kavaklí Akkaş, P. and O. Güven, “Removal of Concentrated Heavy Metal Ions from Aqueous Solutions Using Polymers with Enriched Amidoxime Groups,” J. Appl. Polym. Sci., 93, 1705–1710 (2004c).
Kise, H. and H. Sato, “Synthesis of a New Chelate Resin for Uranium Adsorption from Seawater. Polystyrene Resin Containing Two Amidoxime Functions in the Repeating Unit,” Makromol. Chem., 186, 2449–2454 (1985).
Lutfor, M.R., S. Silong, W.M. Zin, M.Z. Rahman, M. Ahmad, and J. Haron, “Preparation and Characterization of Poly(Amidoxime) Resin from Polyacrylonitrile Grafted Sago Starch,” Eur. Polym. J., 36, 2105–2113 (2000).
Maria, L., M. Amorim, M. Aguiar, P. Guimaraes, M. Costa, A. Aguiar, P. Rezende, M. Carvalho, F. Barbosa, J. Andrade, and R. Riberio, “Chemical Modification of Cross-Linked Resin Based on Acrylonitrile for Anchoring Metal Ions,” React. Funct. Polym., 49, 133–143 (2001).
Park, I.H. and J.M. Suh, “Preparation and Uranyl Ion Adsorptivity of Macroreticular Chelating Resins Containing a Pair of Neighboring Amidoxime Groups in a Monomeric Styrene Unit,” Angew. Makromol. Chem., 239, 121–132 (1996).
Pearson, R.G., “Hard and Soft Acids and Bases,” J. Am. Chem. Soc., 85, 3533–3539 (1963).
Pekel, N., N. şahiner, P. Akkaş, and O. Güven, “Uranyl Ion Adsorptivity of from N-Vinyl 2-pyrrolidone/acrylonitrile Copolymeric Hydrogels Containing Amidoxime group,” Polym. Bull., 44, 593–600 (2000).
Pletner, I.V. and V.V. Zernov, “Classification of Metal Ions According to their Complexing Properties: A Data-Driven Approach,” Anal. Chim. Acta, 455, 131–142 (2002).
Riqueza, E.C., A. Aguiar, L. Maria, and M. Aguiar, “Modification of Porous Copolymers Network Based on Acrylonitrile,” Polym. Bull., 48, 407–414 (2002).
Rivas, B.L., A. Hernan, A. Maturana, and S. Villegas, “Adsorption Behavior of Metal Ions by Amidoxime Chelating Resin,” J. Appl. Polm. Sci., 77, 1994–1999 (2000).
Rivas, B.L., S.A. Pooley, H.A. Maturana, and S. Villegas, “Metal Ion Uptake Properties of Acrylamide Derivative Resins,” Macromol. Chem. Phys., 202, 443–447 (2001).
Sahni, S.K. and J. Reedijk, “Coordination Chemistry of Chelating Resins and Ion Exchangers,” Coor. Chem. Rev., 59, 1–139 (1984).
Saito, K. and T. Sugo, Private communication, Mission: Possible Radiation-Induced Graft Polymerization [1983–2000] printed in Japan (2000).
Saito, K. and T.J. Miyauchi, “Diffusivities of Uranium in Artificial Seawater,” Kagaku Kogagu Ronbunshu, 7, 545–548 (1981).
Saito, K. and T.J. Miyauchi, “Chemical Forms of Uranium in Artificial Seawater,” Nucl. Sci. Technol., 19, 145–150 (1982).
Saraydín, D., E. Karadaĝ, and O. Güven, “Acrylamide/Maleic acid Hydrogels,” Polm. Adv. Technol., 6, 719–726 (1995).
şahiner, N., N. Pekel, P. Akkaş, and O. Güven, “Amidoximation and Characterization of New Complexing Hydrogels Prepared from N-Vinyl 2-pyrrolidone/acrylonitrile Systems,” J. M. S. Pure Appl. Chem., 10, 1159–1172 (2000).
Trochimczuk, A.W., B.N. Kolarz, and D.J. Bartkowiak, “Metal Ion Uptake by Ion-Exchange/Chelating Resins Modified with Cyclohexene Oxide and Cyclohexene Sulphide,” Eur. Polym. J., 37, 559–564 (2001).
Zhanhai, Y., R. Lei, and X. Jun, “Synthesis of a New Type of Adsorbent Containing Carboxyl and Amidoxime Groups by Preirradiation Grafting and its Adsorption of Metal Ions,” J. Appl. Polym. Sci., 83, 1986–1992 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavakli, P.A., Seko, N., Tamada, M. et al. A Highly Efficient Chelating Polymer for the Adsorption of Uranyl and Vanadyl Ions at Low Concentrations. Adsorption 10, 309–315 (2005). https://doi.org/10.1007/s10450-005-4816-z
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-005-4816-z