Abstract
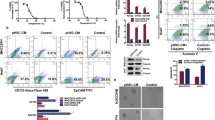
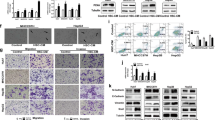
Cryoablation has become a valuable treatment modality for the management of liver cancer. However, one of the major challenges in cryosurgery is the incomplete cryodestruction near the edge of the iceball. This issue can be addressed by optimizing cryoablation parameters and administering thermotropic drugs prior to the procedure. These drugs help enhance tumor response, thereby strengthening the destruction of the incomplete frozen zone in liver cance. In the present study, the feasibility and effectiveness of a thermophysical agent, KCl solution, were investigated to enhance the cryodestruction of HepG2 human liver cancer cells. All cryoablation parameters were simultaneously optimized in order to significantly improve the effect of cryoablation, resulting in an increase in the lethal temperature from − 25 °C to − 17 °C. Subsequently, it was found that the application of KCl solution prior to freezing significantly decreased cell viability post-thaw compared to cryoablation treatment alone. This effect was attributed to the eutectic effect of KCl solution. Importantly, it was found that the combination of KCl solution and freezing was less effective when applied to LO2 human liver normal cells. The data revealed that the ratio of mRNA levels of Bcl-2 and bax decreased significantly more in HepG2 cells than in LO2 cells when cryoablation was used with KCl solution. In conclusion, the results of this study demonstrate the effectiveness of KCl solution in promoting cryoablation and describe a novel therapeutic model for the treatment of liver cancer that may distinguish between cancer and normal cells.







Similar content being viewed by others
References
Anwanwan, D., S. K. Singh, S. Singh, V. Saikam, and R. Singh. Challenges in liver cancer and possible treatment approaches. Biochimica et biophysica acta Rev. Cancer. 1873(1):188314, 2020. https://doi.org/10.1016/j.bbcan.2019.188314.
Li, X., P. Ramadori, D. Pfister, M. Seehawer, L. Zender, and M. Heikenwalder. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer. 21(9):541–557, 2021. https://doi.org/10.1038/s41568-021-00383-9.
Chen, S., Q. Cao, W. Wen, and H. Wang. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett. 460:1–9, 2019. https://doi.org/10.1016/j.canlet.2019.114428.
Gilles, H., T. Garbutt, and J. Landrum. Hepatocellular carcinoma. Crit Care Nurs Clin N. Am. 34(3):289–301, 2022. https://doi.org/10.1016/j.cnc.2022.04.004.
Yan, Q., F. He, B. Q. Wang, et al. Argon-helium cryoablation for liver carcinoma in high-risk locations: safety and efficacy. Cryobiology. 90:8–14, 2019. https://doi.org/10.1016/j.cryobiol.2019.09.006.
Li, X., J. H. Xu, X. Q. Gu, et al. Case report: antiangiogenic therapy plus immune checkpoint inhibitors combined with intratumoral cryoablation for hepatocellular carcinoma. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.740790.
Gage, A. A., and J. G. Baust. Cryosurgery for tumors—a clinical overview. Technol. Cancer Res. Treat. 3(2):187–199, 2004. https://doi.org/10.1177/153303460400300212.
Gage, A. A., and J. Baust. Mechanisms of tissue injury in cryosurgery. Cryobiology. 37(3):171–186, 1998. https://doi.org/10.1006/cryo.1998.2115.
Orlacchio, A., G. Bazzocchi, D. Pastorelli, et al. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: initial experience. Cardiovasc. Interv. Radiol. 31(3):587–594, 2008. https://doi.org/10.1007/s00270-008-9293-9.
Paganini, A. M., A. Rotundo, L. Barchetti, and E. Lezoche. Cryosurgical ablation of hepatic colorectal metastases. Surg. Oncol. 16(Suppl 1):S137–S140, 2007. https://doi.org/10.1016/j.suronc.2007.10.031.
Seifert, J. K., and T. Junginger. Cryotherapy for liver tumors: current status, perspectives, clinical results, and review of literature. Technol. Cancer Res. Treat. 3(2):151–163, 2004. https://doi.org/10.1177/153303460400300208.
Subar, D. A., A. J. Sheen, and D. J. Sherlock. Cryoablation for liver tumors—is there clinical utility? MedGenMed: Medsc. Gener. Med. 5(4):19, 2003.
Shah, T. T., U. Arbel, S. Foss, et al. Modeling cryotherapy ice ball dimensions and isotherms in a novel gel-based model to determine optimal cryo-needle configurations and settings for potential use in clinical practice. Urology. 91:234–240, 2016. https://doi.org/10.1016/j.urology.2016.02.012.
Littrup, P. J., B. Jallad, V. Vorugu, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J. Vasc. Interv. Radiol.: JVIR. 20(10):1343–1351, 2009. https://doi.org/10.1016/j.jvir.2009.05.038.
Baust, J. M., A. Robilotto, K. K. Snyder, et al. Assessment of cryosurgical device performance using a 3D tissue-engineered cancer model. Technol. Cancer Res. Treat. 16(6):900–909, 2017. https://doi.org/10.1177/1533034617708960.
Seifert, J. K., C. D. Gerharz, F. Mattes, et al. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology. 47(3):214–226, 2003. https://doi.org/10.1016/j.cryobiol.2003.10.007.
Larson, T. R., D. W. Rrobertson, A. Corica, and D. G. Bostwick. In vivo interstitial temperature mapping of the human prostate during cryosurgery with correlation to histopathologic outcomes. Urology. 55(4):547–552, 2000. https://doi.org/10.1016/s0090-4295(99)00590-7.
Klossner, D. P., J. M. Baust, R. G. VanBuskirk, A. A. Gage, and J. G. Baust. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 101(10):1310–1316, 2008. https://doi.org/10.1111/j.1464-410X.2008.07499.x.
Klossner, D. P., A. T. Robilotto, D. M. Clarke, et al. Cryosurgical technique: assessment of the fundamental variables using human prostate cancer model systems. Cryobiology. 55(3):189–199, 2007. https://doi.org/10.1016/j.cryobiol.2007.07.003.
Bischof, J., K. Christov, and B. Rubinsky. A morphological study of cooling rate response in normal and neoplastic human liver tissue: cryosurgical implications. Cryobiology. 30(5):482–492, 1993. https://doi.org/10.1006/cryo.1993.1049.
Clarke, D. M., A. T. Robilotto, E. Rhee, et al. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol. Cancer Res. Treat. 6(2):69–79, 2007. https://doi.org/10.1177/153303460700600203.
Baust, J. G., K. K. Snyder, K. L. Santucci, A. T. Robilotto, R. G. Van Buskirk, and J. M. Baust. Cryoablation: physical and molecular basis with putative immunological consequences. Int. J. Hyperth. 36(sup1):10–16, 2019. https://doi.org/10.1080/02656736.2019.1647355.
Tanwar, S., L. Famhawite, and P. R. Verma. Numerical Simulation of bio-heat transfer for cryoablation of regularly shaped tumours in liver tissue using multiprobes. J Therm Biol. 113:103531, 2023. https://doi.org/10.1016/j.jtherbio.2023.103531.
Zhang, X., L. Zheng, K. Suleiman, and C. Shu. Combined cryosurgery and cold-responsive drug-loaded nanoparticles to enhance deep-lying tumor therapy: A mathematical model. Int. J. Heat Mass Transf. 165:120663, 2021. https://doi.org/10.1016/j.ijheatmasstransfer.2020.120663.
Kwak, K., B. Yu, R. J. Lewandowski, and D. H. Kim. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics. 12(5):2175–2204, 2022. https://doi.org/10.7150/thno.67530.
Ou, W., S. Stewart, A. White, et al. In-situ cryo-immune engineering of tumor microenvironment with cold-responsive nanotechnology for cancer immunotherapy. Nat. Commun. 14(1):392, 2023. https://doi.org/10.1038/s41467-023-36045-7.
Clarke, D. M., J. M. Baust, R. G. Van Buskirk, and J. G. Baust. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 42(4):274–285, 2001. https://doi.org/10.1006/cryo.2001.2333.
Clarke, D. M., J. M. Baust, R. G. Van Buskirk, and J. G. Baust. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 49(1):45–61, 2004. https://doi.org/10.1016/j.cryobiol.2004.05.003.
Le Pivert, P., R. S. Haddad, A. Aller, et al. Ultrasound guided combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of human prostate tumors in xenogenic mouse model assessed by luminescence imaging. Technol. Cancer Res. Treat. 3(2):135–142, 2004. https://doi.org/10.1177/153303460400300206.
Baust, J. G., J. C. Bischof, S. Jiang-Hughes, et al. Re-purposing cryoablation: a combinatorial “therapy” for the destruction of tissue. Prostate Cancer Prostatic Dis. 18(2):87–95, 2015. https://doi.org/10.1038/pcan.2014.54.
Koushafar, H., L. Pham, C. Lee, and B. Rubinsky. Chemical adjuvant cryosurgery with antifreeze proteins. J. Surg. Oncol. 66(2):114–121, 1997. https://doi.org/10.1002/(sici)1096-9098(199710)66:2%3c114::aid-jso8%3e3.0.co;2-g.
Goel, R., K. Anderson, J. Slaton, et al. Adjuvant approaches to enhance cryosurgery. J. Biomech. Eng. 131(7):074003, 2009. https://doi.org/10.1115/1.3156804.
Han, B., A. Iftekhar, and J. C. Bischof. Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol. Cancer Res. Treat. 3(2):103–111, 2004. https://doi.org/10.1177/153303460400300203.
Wang, C. L., K. Y. Teo, and B. Han. An amino acidic adjuvant to augment cryoinjury of MCF-7 breast cancer cells. Cryobiology. 57(1):52–59, 2008. https://doi.org/10.1016/j.cryobiol.2008.05.007.
Qiu, Q., L. Jiang, H. Zhen, et al. Promotion of HepG2 cell apoptosis by Sedum emarginatum Migo and the mechanism of action. BMC Complement. Med. Ther. 22(1):31, 2022. https://doi.org/10.1186/s12906-022-03503-6.
Yang, F., R. Pei, Z. Zhang, et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. In Vitro. 54:310–316, 2019. https://doi.org/10.1016/j.tiv.2018.10.017.
Claro, S., M. E. Oshiro, R. A. Mortara, et al. γ-Rays-generated ROS induce apoptosis via mitochondrial and cell cycle alteration in smooth muscle cells. Int. J. Radiat. Biol. 90(10):914–927, 2014. https://doi.org/10.3109/09553002.2014.911988.
Livak, K. J., and T. D. Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25(4):402–408, 2001. https://doi.org/10.1006/meth.2001.1262.
Baust, J. G., and A. A. Gage. The molecular basis of cryosurgery. BJU Int. 95(9):1187–1191, 2005. https://doi.org/10.1111/j.1464-410X.2005.05502.x.
Rubinsky, B. Cryosurgery. Annu. Rev. Biomed. Eng. 2:157–187, 2000. https://doi.org/10.1146/annurev.bioeng.2.1.157.
Liu, W., Z. Huang, X. He, et al. Impacts of trehalose and l-proline on the thermodynamic nonequilibrium phase change and thermal properties of normal saline. Cryobiology. 96:92–98, 2020. https://doi.org/10.1016/j.cryobiol.2020.07.011.
Adams, J. M., and S. Cory. The Bcl-2 protein family: arbiters of cell survival. Science. 281(5381):1322–1326, 1998. https://doi.org/10.1126/science.281.5381.1322.
Guo, X., N. Yang, W. Ji, et al. Mito-bomb: targeting mitochondria for cancer therapy. Adv. Mater. 33(43):e2007778, 2021. https://doi.org/10.1002/adma.202007778.
Herr, I., and K. M. Debatin. Cellular stress response and apoptosis in cancer therapy. Blood. 98(9):2603–2614, 2001. https://doi.org/10.1182/blood.v98.9.2603.
Mala, T., L. Aurdal, L. Frich, et al. Liver tumor cryoablation: a commentary on the need of improved procedural monitoring. Technol. Cancer Res. Treat. 3(1):85–91, 2004. https://doi.org/10.1177/153303460400300110.
He, X., Y. Xiao, X. Zhang, et al. Percutaneous tumor ablation: cryoablation facilitates targeting of free epirubicin-ethanol-ioversol solution interstitially coinjected in a rabbit VX2 tumor model. Technol. Cancer Res. Treat. 15(4):597–608, 2016. https://doi.org/10.1177/1533034615593855.
Moore, G. E., R. E. Gerner, and H. A. Franklin. Culture of normal human leukocytes. JAMA. 199(8):519–524, 1967.
Han, B., and J. C. Bischof. Direct cell injury associated with eutectic crystallization during freezing. Cryobiology. 48(1):8–21, 2004. https://doi.org/10.1016/j.cryobiol.2003.11.002.
Bischof, J. C., D. Smith, P. V. Pazhayannur, C. Manivel, J. Hulbert, and K. P. Roberts. Cryosurgery of dunning AT-1 rat prostate tumor: thermal, biophysical, and viability response at the cellular and tissue level. Cryobiology. 34(1):42–69, 1997. https://doi.org/10.1006/cryo.1996.1978.
Kumari, C., A. Kumar, S. K. Sarangi, and A. Thirugnanam. Effect of adjuvant on cutaneous cryotherapy. Heat Mass Transf. 55(2):247–260, 2019.
Lang, F., M. Föller, K. S. Lang, et al. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 205(3):147–157, 2005. https://doi.org/10.1007/s00232-005-0780-5.
Bilney, A. J., and A. W. Murray. Pro- and anti-apoptotic effects of K+ in HeLa cells. FEBS Lett. 424(3):221–224, 1998. https://doi.org/10.1016/s0014-5793(98)00172-0.
Wang, X., W. P. Liu, X. Zhang, et al. Effect of extracellular K+ on the apoptosis of neural stem cells. Chin. J. Stereotact. Funct. Neurosurg. 04:216–219, 2008.
Zaib, S., A. Hayyat, N. Ali, A. Gul, M. Naveed, and I. Khan. Role of mitochondrial membrane potential and lactate dehydrogenase A in apoptosis. Anti-Cancer Agents Med. Chem. 22(11):2048–2062, 2022. https://doi.org/10.2174/1871520621666211126090906.
Allemani, C., T. Matsuda, V. Di Carlo, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 391(10125):1023–1075, 2018. https://doi.org/10.1016/s0140-6736(17)33326-3.
Song, X. D., J. J. Zhang, M. R. Wang, W. B. Liu, X. B. Gu, and C. J. Lv. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 34(6):839–844, 2011. https://doi.org/10.1248/bpb.34.839.
Hou, Y., X. Sun, M. Dou, C. Lu, J. Liu, and W. Rao. Cellulose nanocrystals facilitate needle-like ice crystal growth and modulate molecular targeted ice crystal nucleation. Nano Lett. 21(11):4868–4877, 2021. https://doi.org/10.1021/acs.nanolett.1c00514.
Acknowledgments
The project was supported by the Shanghai Collaborative Innovation Center for Tumor Treatment with Energy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis, original draft preparation: [MC]; review and editing: [WL]; funding acquisition, resources, and supervision: [BL]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
No conflicting financial interests exist.
Ethical Approval
Our study did not require an ethical board approval because it did not contain human or animal trials.
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Liu, W. & Liu, B. Cryoablation with KCl Solution Enhances Necrosis and Apoptosis of HepG2 Liver Cancer Cells. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03512-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03512-1