Abstract
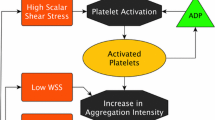
The unacceptably high stroke rate associated with HeartMate 3 ventricular assist device (VAD) without signs of adherent pump thrombosis is hypothesized to be the result of the emboli produced by the inflow cannula, that are ingested and ejected from the pump. This in vitro and numerical study aimed to emulate the surface features and supraphysiological shear of a ventricular cannula to provide insight into their effect on thrombogenesis. Human whole blood was perfused at calibrated flow rates in a microfluidic channel to achieve shear rates 1000–7500 s−1, comparable to that experienced on the cannula. The channel contained periodic teeth representative of the rough sintered surface of the HeartMate 3 cannula. The deposition of fluorescently labeled platelets was visualized in real time and analyzed with a custom entity tracking algorithm. Numerical simulations of a multi-constituent thrombosis model were performed to simulate laminar blood flow in the channel. The sustained growth of adherent platelets was observed in all shear conditions (\(p<\) 0.05). However, the greatest deposition was observed at the lower shear rates. The location of deposition with respect to the microfluidic teeth was also found to vary with shear rate. This was confirmed by CFD simulation. The entity tracking algorithm revealed the spatial variation of instances of embolic events. This result suggests that the sintered surface of the ventricular cannula may engender unstable thrombi with a greater likelihood of embolization at supraphysiological shear rates.









Similar content being viewed by others
References
Maher, T. R., K. C. Butler, V. L. Poirier, and D. B. Gernes. HeartMate left ventricular assist devices: a multigeneration of implanted blood pumps. Artif. Organs. 25(5):422–426, 2001. https://doi.org/10.1046/j.1525-1594.2001.06756.x.
Starling, R. C., N. Moazami, S. C. Silvestry, G. Ewald, J. G. Rogers, C. A. Milano, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N. Engl. J. Med. 370(1):33–40, 2014. https://doi.org/10.1056/NEJMOA1313385.
Cho, S. M., P. Tahsili-Fahadan, A. Kilic, C. W. Choi, R. C. Starling, and K. Uchino. A comprehensive review of risk factor, mechanism, and management of left ventricular assist device-associated stroke. Semin. Neurol. 41(4):411–421, 2021. https://doi.org/10.1055/s-0041-1726328.
Rogers, J. G., F. D. Pagani, A. J. Tatooles, G. Bhat, M. S. Slaughter, E. J. Birks, et al. Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 376(5):451–460, 2017. https://doi.org/10.1056/nejmoa1602954.
Stehlik, J., and J. K. Kirklin. The long and winding road to an effective left ventricular assist device: the demise of Medtronic’s HVAD. Circulation. 144(7):509–511, 2021. https://doi.org/10.1161/CIRCULATIONAHA.121.056027.
Colombo, P. C., M. R. Mehra, D. J. Goldstein, J. D. Estep, C. Salerno, U. P. Jorde, et al. Comprehensive analysis of stroke in the long-term cohort of the MOMENTUM 3 study: a randomized controlled trial of the HeartMate 3 versus the HeartMate II cardiac pump. Circulation. 139(2):155–168, 2019. https://doi.org/10.1161/CIRCULATIONAHA.118.037231.
Glass, C. H., A. Christakis, G. A. Fishbein, J. C. Watkins, K. C. Strickland, R. N. Mitchell, et al. Thrombus on the inflow cannula of the HeartWare HVAD: an update. Cardiovasc. Pathol. 38:14–20, 2019. https://doi.org/10.1016/j.carpath.2018.09.002.
Mehra, M. R., N. Uriel, Y. Naka, J. C. Cleveland, M. Yuzefpolskaya, C. T. Salerno, et al. A fully magnetically levitated left ventricular assist device—final report. N. Engl. J. Med. 380(17):1618–1627, 2019. https://doi.org/10.1056/NEJMOA1900486.
Li, S., J. A. Beckman, R. Cheng, C. Ibeh, C. J. Creutzfeldt, J. Bjelkengren, et al. Comparison of neurologic event rates among HeartMate II, HeartMate 3, and HVAD. ASAIO J. 66(6):620–624, 2020. https://doi.org/10.1097/MAT.0000000000001084.
Rowlands, G. W., and J. F. Antaki. High-speed visualization of ingested, ejected, adherent, and disintegrated thrombus in contemporary ventricular assist devices. Artif. Organs. 44(11):E459–E469, 2020. https://doi.org/10.1111/aor.13753.
Griffith, B. P., R. L. Kormos, H. S. Borovetz, K. Litwak, J. F. Antaki, V. L. Poirier, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann. Thorac. Surg. 2001. https://doi.org/10.1016/S0003-4975(00)02639-4.
Bakir, M. Haemocompatibility of titanium and its alloys. J. Biomater. Appl. 27(1):3–15, 2012. https://doi.org/10.1177/0885328212439615.
Jamiolkowski, M. A., J. R. Woolley, M. V. Kameneva, J. F. Antaki, and W. R. Wagner. Real time visualization and characterization of platelet deposition under flow onto clinically relevant opaque surfaces. J. Biomed. Mater. Res. A. 103(4):1303–1311, 2015. https://doi.org/10.1002/jbm.a.35202.
He, W., J. T. Butcher, G. W. Rowlands, and J. F. Antaki. Biological response to sintered titanium in left ventricular assist devices: pseudoneointima, neointima, and pannus. ASAIO J. 2022. https://doi.org/10.1097/mat.0000000000001777.
Bagot, C. N., and R. Arya. Virchow and his triad: a question of attribution. Br. J. Haematol. 143(2):180–190, 2008. https://doi.org/10.1111/j.1365-2141.2008.07323.x.
Wiegmann, L., B. Thamsen, D. de Zélicourt, M. Granegger, S. Boës, M. Schmid Daners, et al. Fluid dynamics in the HeartMate 3: influence of the artificial pulse feature and residual cardiac pulsation. Artif. Organs. 43(4):363–376, 2019. https://doi.org/10.1111/aor.13346.
Méndez Rojano, R., M. Zhussupbekov, and J. F. Antaki. Multi-constituent simulation of thrombus formation at LVAD inlet cannula connection: importance of Virchow’s triad. Artif. Organs 45(9):1014–1023, 2021. https://doi.org/10.1111/aor.13949. arXiv:2011.10479.
Jayaraman, A., J. Kang, J. F. Antaki, and B. J. Kirby. The roles of sub-micron and microscale roughness on shear-driven thrombosis on titanium alloy surfaces. Artif. Organs. 2022. https://doi.org/10.1111/aor.14467.
Jamiolkowski, M. A., D. D. Pedersen, W. T. Wu, J. F. Antaki, and W. R. Wagner. Visualization and analysis of biomaterial-centered thrombus formation within a defined crevice under flow. Biomaterials. 96:72–83, 2016. https://doi.org/10.1016/j.biomaterials.2016.04.022.
Castrodeza, J., C. Ortiz-Bautista, and F. Fernández-Avilés. Continuous-flow left ventricular assist device: current knowledge, complications, and future directions. Cardiol. J. 29(2):293–304, 2022. https://doi.org/10.5603/CJ.a2021.0172.
Dasse, K. A., S. D. Chipman, C. N. Sherman, A. H. Levine, and O. H. Frazier. Clinical experience with textured blood contacting surfaces in ventricular assist devices. ASAIO Trans. 33(3):418–425, 1987.
George-Gay, B., and K. Parker. Understanding the complete blood count with differential. J. Perianesth. Nurs. 18(2):96–117, 2003. https://doi.org/10.1053/jpan.2003.50013.
Aizawa, H., H. Kawabata, A. Sato, H. Masuki, T. Watanabe, T. Tsujino, et al. A comparative study of the effects of anticoagulants on pure platelet-rich plasma quality and potency. Biomedicines. 8(3):1–14, 2020. https://doi.org/10.3390/biomedicines8030042.
Johnson, C. A., S. Vandenberghe, A. R. Daly, J. R. Woolley, S. T. Snyder, J. E. Verkaik, et al. Biocompatibility assessment of the first generation PediaFlow pediatric ventricular assist device. Artif. Organs. 35(1):9–21, 2011. https://doi.org/10.1111/j.1525-1594.2010.01023.x.
Cohen-Mansfield, J., M. Dakheel-Ali, M. S. Marx, K. Thein, N. G. Regier, et al. Impact of LVAD implantation site on ventricular blood stagnation. Physiol. Behav. 176(1):139–148, 2017. https://doi.org/10.1097/MAT.0000000000000503.Impact.
Chivukula, V. K., J. A. Beckman, A. R. Prisco, T. Dardas, S. Lin, J. W. Smith, et al. Left ventricular assist device inflow cannula angle and thrombosis risk. Circ.: Heart Fail. 11(4):1–8, 2018. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004325.
Chivukula, V. K., J. A. Beckman, A. R. Prisco, S. Lin, T. F. Dardas, R. K. Cheng, et al. Small left ventricular size is an independent risk factor for ventricular assist device thrombosis. ASAIO J. 65(2):152–159, 2019. https://doi.org/10.1097/MAT.0000000000000798.
Kang, J., A. Jayaraman, J. F. Antaki, and B. J. Kirby. Extracting mural and volumetric growth patterns of platelet aggregates on engineered surfaces by use of an entity tracking algorithm. ASAIO J. 69(4):382–390, 2023. https://doi.org/10.1097/MAT.0000000000001841.
Zhussupbekov, M., R. M. Rojano, W. T. Wu, and J. F. Antaki. von Willebrand factor unfolding mediates platelet deposition in a model of high-shear thrombosis. Biophys. J. 121(21):4033–4047, 2022. https://doi.org/10.1016/j.bpj.2022.09.040. arXiv:2204.04305.
Wu, W. T., M. A. Jamiolkowski, W. R. Wagner, N. Aubry, M. Massoudi, and J. F. Antaki. Multi-constituent simulation of thrombus deposition. Sci. Rep. 7(1):1–16, 2017. https://doi.org/10.1038/srep42720. arXiv:1601.06717.
Zhussupbekov, M., R. Méndez Rojano, W. T. Wu, M. Massoudi, and J. F. Antaki. A continuum model for the unfolding of von Willebrand factor. Ann. Biomed. Eng. 49(9):2646–2658, 2021. https://doi.org/10.1007/s10439-021-02845-5.
Ruggeri, Z. M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 2007. https://doi.org/10.1016/j.thromres.2007.03.011.
Rana, A., E. Westein, B. Niego, and C. E. Hagemeyer. Shear-dependent platelet aggregation: mechanisms and therapeutic opportunities. Front. Cardiovasc. Med. 6:141, 2019. https://doi.org/10.3389/fcvm.2019.00141.
Eulert-Grehn, J. J., T. Krabatsch, and E. Potapov. A case of an obstructive inflow thrombus in a HeartMate 3 from the left ventricle into the pump. J. Heart Lung Transplant. 37(1):172–173, 2018. https://doi.org/10.1016/J.HEALUN.2017.07.025.
Netuka, I., and M. R. Mehra. Ischemic stroke and subsequent thrombosis within a HeartMate 3 left ventricular assist system: a cautionary tale. J. Heart Lung Transplant. 37(1):170–172, 2018. https://doi.org/10.1016/j.healun.2017.11.002.
Han, Q., S. M. Shea, T. Arleo, J. Y. Qian, and D. N. Ku. Thrombogenicity of biomaterials depends on hemodynamic shear rate. Artif. Organs. 46(4):606–617, 2022. https://doi.org/10.1111/aor.14093.
Jamiolkowski, M. A., M. C. Hartung, R. A. Malinauskas, and Q. Lu. An in vitro blood flow loop system for evaluating the thrombogenicity of medical devices and biomaterials. ASAIO J. 2020. https://doi.org/10.1097/MAT.0000000000000958.
Zhussupbekov, M., W. T. Wu, M. A. Jamiolkowski, M. Massoudi, and J. F. Antaki. Influence of shear rate and surface chemistry on thrombus formation in micro-crevice. J. Biomech. 121:110397, 2021. https://doi.org/10.1016/j.jbiomech.2021.110397.
Thierry, B., Y. Merhi, L. Bilodeau, C. Trépanier, and M. Tabrizian. Nitinol versus stainless steel stents: acute thrombogenicity study in an ex vivo porcine model. Biomaterials. 23(14):2997–3005, 2002. https://doi.org/10.1016/S0142-9612(02)00030-3.
Litvinov, R. I., and J. W. Weisel. Blood clot contraction: mechanisms, pathophysiology, and disease. Res. Pract. Thromb. Haemost. 7(1):100023, 2023. https://doi.org/10.1016/j.rpth.2022.100023.
Tunströmer, K., L. Faxälv, N. Boknäs, and T. L. Lindahl. Quantification of platelet contractile movements during thrombus formation. Thromb. Haemost. 118(9):1600–1611, 2018. https://doi.org/10.1055/s-0038-1668151.
Savage, B., E. Saldívar, and Z. M. Ruggeri. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 84(2):289–297, 1996. https://doi.org/10.1016/S0092-8674(00)80983-6.
Michelson, A. D., M. R. Barnard, L. A. Krueger, A. L. Frelinger, and M. I. Furman. Evaluation of platelet function by flow cytometry. Methods. 21(3):259–270, 2000. https://doi.org/10.1006/meth.2000.1006.
Sharda, A., and R. Flaumenhaft. The life cycle of platelet granules. F1000Research. 7(236):1–12, 2018.
Barić, D. Why pulsatility still matters: a review of current knowledge. Croat. Med. J. 55(6):609–620, 2014. https://doi.org/10.3325/cmj.2014.55.609.
May-Newman, K., N. Marquez-Maya, R. Montes, and S. Salim. The effect of inflow cannula angle on the intraventricular flow field of the left ventricular assist device-assisted heart: an in vitro flow visualization study. ASAIO J. 65(2):139–147, 2019. https://doi.org/10.1097/MAT.0000000000000790.
Ortiz, S., V. Vu, R. Montes, and K. May-Newman. Left ventricular flow dynamics with the HeartMate3 left ventricular assist device: effect of inflow cannula position and speed modulation. ASAIO J. 67(12):1301–1311, 2021. https://doi.org/10.1097/MAT.0000000000001523.
Acknowledgements
We would like to extend our gratitude to the National Institute of Health Grant HL089456 for funding this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 2 (MP4 50827 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, W., Karmakar, A., Kang, J. et al. In Vitro and In Silico Characterization of the Aggregation of Thrombi on Textured Ventricular Cannula. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03504-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03504-1