Abstract
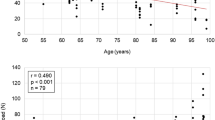
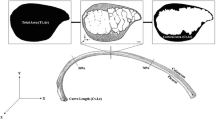
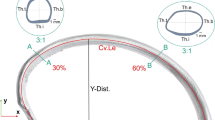
The use of ovine animal models in the study of injury biomechanics and modeling is increasing, due to their favorable size and other physiological characteristics. Along with this increase, there has also been increased interest in the development of in silico ovine models for computational studies to compliment physical experiments. However, there remains a gap in the literature characterizing the morphological and mechanical characteristics of ovine ribs. The objective of this study therefore is to report anatomical and mechanical properties of the ovine ribs using microtomography (micro-CT) and two types of mechanical testing (quasi-static bending and dynamic tension). Using microtomography, young ovine rib samples obtained from a local abattoir were cut into approximately fourteen 38 mm sections and scanned. From these scans, the cortical bone thickness and cross-sectional area were measured, and the moment of inertia was calculated to enhance the mechanical testing data. Based on a standard least squares statistical model, the cortical bone thickness varied depending on the region of the cross-section and the position along the length of the rib (p < 0.05), whereas the cross-sectional area remained consistent (p > 0.05). Quasi-static three-point bend testing was completed on ovine rib samples, and the resulting force–displacement data was analyzed to obtain the stiffness (44.67 ± 17.65 N/mm), maximum load (170.54 ± 48.28 N) and displacement at maximum load (7.19 ± 2.75 mm), yield load (167.81 ± 48.12 N) and displacement at yield (6.10 ± 2.25 mm), and the failure load (110.90 ± 39.30 N) and displacement at failure (18.43 ± 2.10 mm). The resulting properties were not significantly affected by the rib (p > 0.05), but by the animal they originated from (p < 0.05). For the dynamic testing, samples were cut into coupons and tested in tension with an average strain rate of 18.9 strain/sec. The resulting dynamic testing properties of elastic modulus (5.16 ± 2.03 GPa), failure stress (63.29 ± 14.02 MPa), and failure strain (0.0201 ± 0.0052) did not vary based on loading rate (p > 0.05).








Similar content being viewed by others
References
Appleyard, R. C., et al. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthr. Cartil. 11(1):65–77, 2003. https://doi.org/10.1053/joca.2002.0867.
Bass, C. R., et al. Injury risk in behind armor blunt thoracic trauma. Int. J. Occup. Saf. Ergon. 12(4):429–442, 2006. https://doi.org/10.1080/10803548.2006.11076702.
Biasutti, S., et al. Spatiotemporal variations in gene expression, histology and biomechanics in an ovine model of tendinopathy. PLoS ONE. 12(10):e0185282, 2017. https://doi.org/10.1371/journal.pone.0185282.
Brogden, K. A., V. C. Kalfa, M. R. Ackermann, D. E. Palmquist, P. B. McCray Jr., and B. F. Tack. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob. Agents Chemother. 45(1):331–334, 2001. https://doi.org/10.1128/AAC.45.1.331-334.2001.
Daryabari, S. S., et al. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 36(6):1211–1223, 2019.
Easley, N. E., M. Wang, L. M. McGrady, and J. M. Toth. Biomechanical and radiographic evaluation of an ovine model for the human lumbar spine. Proc. Inst. Mech. Eng. H. 222(6):915–922, 2008. https://doi.org/10.1243/09544119JEIM345.
FSIS. Lamb from Farm to Table. (2020).
Gibbons, M. M., X. Dang, M. Adkins, B. Powell, and P. Chan. Finite element modeling of blast lung injury in sheep. J. Biomech. Eng. 137(4):041002, 2015. https://doi.org/10.1115/1.4029181.
Gleiser, I., and E. Hunt. The estimation of age and sex of preadolescent children from bones and teeth. J. Am. J. Phys. Anthr. 13:479–488, 1955.
Goldfarb, M. A., T. F. Ciurej, M. A. Weinstein, and L. W. Metker. "A method for soft body armor evaluation: medical assessment. EDGEWOOD ARSENAL ABERDEEN PROVING GROUND MD, 1975.
Greer, A., D. Cronin, C. Salisbury, and K. Williams. Finite element modeling for the prediction of blast trauma. In: IUTAM Symposium on Impact Biomechanics: From Fundamental Insights to Applications, Springer, 2005, pp. 263–271.
Hanlon, E., and P. Gillich. Origin of the 44-mm behind-armor blunt trauma standard. Mil. Med. 177(3):333–339, 2012. https://doi.org/10.7205/milmed-d-11-00303.
Harris, A. Towards an ovine model of cystic fibrosis. Hum. Mol. Genet. 6(13):2191–2194, 1997. https://doi.org/10.1093/hmg/6.13.2191.
Holcombe, S. A., Y. S. Kang, B. A. Derstine, S. C. Wang, and A. M. Agnew. “Regional maps of rib cortical bone thickness and cross-sectional geometry,” (in eng). J. Anatomy. 235(5):883–891, 2019. https://doi.org/10.1111/joa.13045.
Hurtig, M. B., et al. Preclinical studies for cartilage repair: recommendations from the international cartilage repair society. Cartilage. 2(2):137–152, 2011. https://doi.org/10.1177/1947603511401905.
Johnson, D. L., J. T. Yelverton, W. Hicks, and R. Doyal. Blast overpressure studies with animals and man: biological response to complex blast waves. EG and G INC ALBUQUERQUE NM, 1993.
Katzenberger, M. J., D. L. Albert, A. M. Agnew, and A. R. Kemper. Effects of sex, age, and two loading rates on the tensile material properties of human rib cortical bone. J. Mech. Behav. Biomed. Mater. 102:103410, 2020. https://doi.org/10.1016/j.jmbbm.2019.103410.
A. R. Kemper, C. McNally, C. A. Pullins, L. J. Freeman, S. M. Duma, and S. W. J. S. c. c. j. Rouhana, The biomechanics of human ribs: material and structural properties from dynamic tension and bending tests, vol. 51, p. 235, 2007.
Lau, A., M. L. Oyen, R. W. Kent, D. Murakami, and T. Torigaki. Indentation stiffness of aging human costal cartilage. Acta Biomater. 4(1):97–103, 2008. https://doi.org/10.1016/j.actbio.2007.06.008.
Li, Z., et al. Rib fractures under anterior–posterior dynamic loads: experimental and finite-element study. J. Biomech. 43(2):228–234, 2010.
Li, S., E. Demirci, and V. V. Silberschmidt. Variability and anisotropy of mechanical behavior of cortical bone in tension and compression. J. Mech. Behav. Biomed. Mater. 21:109–120, 2013.
MacFadden, L. N., P. C. Chan, K. H. Ho, and J. H. Stuhmiller. A model for predicting primary blast lung injury. J. Trauma Acute Care Surg. 73(5):1121–1129, 2012. https://doi.org/10.1097/TA.0b013e31825c1536.
Matute-Bello, G., C. W. Frevert, and T. R. Martin. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 295(3):L379–L399, 2008. https://doi.org/10.1152/ajplung.00010.2008.
Ministry of Agriculture Food, and Rural Affairs. Factsheet 19–011, AGDEX 434/10. Predicting Lamb Finishing Dates. Mmm. 1:10, 2019.
Moainie, S. L., et al. An ovine model of postinfarction dilated cardiomyopathy. Ann. Thorac. Surg. 74(3):753–760, 2002. https://doi.org/10.1016/s0003-4975(02)03827-4.
Moreno, J., and F. Forriol. Effects of preservation on the mechanical strength and chemical composition of cortical bone: an experimental study in sheep femora. Biomaterials. 23(12):2615–2619, 2002. https://doi.org/10.1016/s0142-9612(01)00402-1.
Nguyen, T. V., et al. Sex differences in bone mass acquisition during growth: the fels longitudinal study. J. Clin. Densitom. 4(2):147–157, 2001. https://doi.org/10.1385/JCD:4:2:147.
D. O'Brien and S. Wideius. Preparing for the breeding season in meat goats and hair sheep. (2018). Virginia Cooperative Extension. Virginia Tech.
Oftadeh, R., M. Perez-Viloria, J. C. Villa-Camacho, A. Vaziri, and A. Nazarian. Biomechanics and mechanobiology of trabecular bone: a review (in eng). J. Biomech. Eng. 137(1):0108021–01080215, 2015. https://doi.org/10.1115/1.4029176.
Ottenio, M., D. Tran, A. NiAnnaidh, M. D. Gilchrist, and K. Bruyere. Strain rate and anisotropy effects on the tensile failure characteristics of human skin. J. Mech. Behav. Biomed. Mater. 41:241–50, 2015. https://doi.org/10.1016/j.jmbbm.2014.10.006.
Pezowicz, C., and M. Glowacki. The mechanical properties of human ribs in young adult. Acta Bioeng. Biomech. 14(2):53–60, 2012.
Prevrhal, S., K. Engelke, and W. A. Kalender. Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys. Med. Biol. 44(3):751–764, 1999. https://doi.org/10.1088/0031-9155/44/3/017.
Reichert, J. C., et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng. Part B. 16(1):93–104, 2010. https://doi.org/10.1089/ten.TEB.2009.0455.
Rynkevic, R., P. Martins, L. Hympanova, H. Almeida, A. A. Fernandes, and J. Deprest. Biomechanical and morphological properties of the multiparous ovine vagina and effect of subsequent pregnancy. J. Biomech. 57:94–102, 2017. https://doi.org/10.1016/j.jbiomech.2017.03.023.
Thomas, P. K., L. K. Sullivan, G. H. Dickinson, C. M. Davis, and A. G. Lau. The effect of helium ion radiation on the material properties of bone. Calcif. Tissue Int. 108(6):808–818, 2021. https://doi.org/10.1007/s00223-021-00806-7.
Ulrich, D., et al. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PLoS ONE. 9(4):e93172, 2014. https://doi.org/10.1371/journal.pone.0093172.
Wei, A., et al. BMP13 prevents the effects of annular injury in an ovine model. Int. J. Biol. Sci. 5(5):388–396, 2009. https://doi.org/10.7150/ijbs.5.388.
Wilson, S., et al. An ovine model of spinal cord injury. J. Spinal Cord. Med. 40(3):346–360, 2017. https://doi.org/10.1080/10790268.2016.1222475.
Acknowledgments
We would like to acknowledge Veryst Engineering, LLC for running the high-rate tests and the DEVCOM Army Research Lab for funding this project (#W911NF2120034).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Moment of inertia table.
CT Scan | Recon | Long, outer (a) | Short, outer (b) | Long, inner (c) | Short, INNER (d) | MOI (mm4) |
|---|---|---|---|---|---|---|
Rib 3 Scan 4 | 2 | 6.14 | 1.76 | 5.71 | 0.82 | 200.1 |
Rib 4 Scan 7 | 2 | 5.82 | 1.84 | 5.51 | 1.28 | 116.72 |
Rib 5 Scan 10 | 2 | 4.82 | 1.82 | 4.48 | 1.07 | 84.5 |
Rib 6 Scan 13 | 2 | 5.27 | 1.87 | 4.87 | 1.07 | 117.9 |
Median | 117.31 | |||||
Standard Deviation | 49.35206 | |||||
Mean | 129.805 |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, P.K., Caffrey, J., Afetse, K.E. et al. Micro-CT Imaging and Mechanical Properties of Ovine Ribs. Ann Biomed Eng 51, 1513–1522 (2023). https://doi.org/10.1007/s10439-023-03156-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03156-7