Abstract
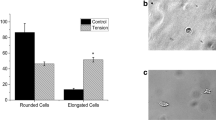
After muscle loss or injury, skeletal muscle tissue has the ability to regenerate and return its function. However, large volume defects in skeletal muscle tissue pose a challenge to regenerate due to the absence of regenerative elements such as biophysical and biochemical cues, making the development of new treatments necessary. One potential solution is to utilize electroactive polymers that can change size or shape in response to an external electric field. Poly(ethylene glycol) diacrylate (PEGDA) is one such polymer, which holds great potential as a scaffold for muscle tissue regeneration due to its mechanical properties. In addition, the versatile chemistry of this polymer allows for the conjugation of new functional groups to enhance its electroactive properties and biocompatibility. Herein, we have developed an electroactive copolymer of PEGDA and acrylic acid (AA) in combination with collagen methacrylate (CMA) to promote cell adhesion and proliferation. The electroactive properties of the CMA + PEGDA:AA constructs were investigated through actuation studies. Furthermore, the biological properties of the hydrogel were investigated in a 14-day in vitro study to evaluate myosin light chain (MLC) expression and metabolic activity of C2C12 mouse myoblast cells. The addition of CMA improved some aspects of material bioactivity, such as MLC expression in C2C12 mouse myoblast cells. However, the incorporation of CMA in the PEGDA:AA hydrogels reduced the sample movement when placed under an electric field, possibly due to steric hindrance from the CMA. Further research is needed to optimize the use of CMA in combination with PEGDA:AA as a potential scaffold for skeletal muscle tissue engineering.











Similar content being viewed by others
References
Agley, C. C., C. P. Velloso, N. R. Lazarus, and S. D. Harridge. An image analysis method for the precise selection and quantitation of fluorescently labeled cellular constituents: application to the measurement of human muscle cells in culture. J. Histochem. Cytochem. 60(6):428–438, 2012. https://doi.org/10.1369/0022155412442897.
Apsite, I., J. M. Uribe, A. F. Posada, S. Rosenfeldt, S. Salehi, and L. Ionov. 4D biofabrication of skeletal muscle microtissues. Biofabrication.12:015016, 2019.
Bach, A. D., J. P. Beier, J. Stern-Staeter, and R. E. Horch. Skeletal muscle tissue engineering. J. Cell. Mol. Med. 8(4):413–422, 2004.
Bar-Cohen, Y. Electroactive polymer (EAP) actuators as artificial muscles: reality, potential, and challenges, 2nd ed. Bellingham, WA: SPIE Press, p. xvii, 2004.
Bar-Cohen, Y. Electroactive polymers as an enabling materials technology. Proc. Inst. Mech. Eng. G. 221(4):553–564, 2007.
Bauer, A., L. Gu, B. Kwee, W. A. Li, M. Dellacherie, A. D. Celiz, et al. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater. 62:82–90, 2017.
Beier, J. P., J. Stern-Straeter, V. T. Foerster, U. Kneser, G. B. Stark, and A. D. Bach. Tissue engineering of injectable muscle: three-dimensional myoblast-fibrin injection in the syngeneic rat animal model. Plast. Reconstr. Surg. 118(5):1113–1121, 2006.
Browe, D. P., C. Wood, M. T. Sze, K. A. White, T. Scott, R. M. Olabisi, et al. Characterization and optimization of actuating poly(ethylene glycol) diacrylate/acrylic acid hydrogels as artificial muscles. Polymer. 117:331–341, 2017.
Cai, A., M. Hardt, P. Schneider, R. Schmid, C. Lange, D. Dippold, et al. Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL–collagen I-nanoscaffolds. BMC Biotechnol. 18(1):1–12, 2018.
Chaturvedi, V., D. Naskar, B. F. Kinnear, E. Grenik, D. E. Dye, M. D. Grounds, et al. Silk fibroin scaffolds with muscle-like elasticity support in vitro differentiation of human skeletal muscle cells. J. Tissue Eng. Regen. Med. 11:3178–3192, 2017.
Chaubaroux, C., F. Perrin-Schmitt, B. Senger, L. Vidal, J. C. Voegel, P. Schaaf, et al. Cell alignment driven by mechanically induced collagen fiber alignment in collagen/alginate coatings. Tissue Eng. C. 21(9):881–888, 2015.
Chen, S., T. Nakamoto, N. Kawazoe, and G. Chen. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials. 73:23–31, 2015. https://doi.org/10.1016/j.biomaterials.2015.09.010.
Choi, Y. J., Y. J. Jun, D. Y. Kim, H. G. Yi, S. H. Chae, J. Kang, et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 206:160–169, 2019.
Choi, Y. J., T. G. Kim, J. Jeong, H. G. Yi, J. W. Park, W. Hwang, et al. 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv. Healthc. Mater. 5(20):2636–2645, 2016.
Clark, M. E. editor Pain issues among OEF and OIF returnees. In: VA/DOD Emerging Concepts Conference, 2007, Las Vegas, NV.
De Deyne, P. G. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am. J. Physiol. Cell Physiol. 279(6):C1801–C1811, 2000.
De Santis, M. M., H. N. Alsafadi, S. Tas, D. A. Bolukbas, S. Prithiviraj, I. A. N. Da Silva, et al. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mater.33:e2005476, 2021.
Denes, L. T., L. A. Riley, J. R. Mijares, J. D. Arboleda, K. McKee, K. A. Esser, et al. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet. Muscle. 9(1):17, 2019.
Drzewiecki, K. E., A. S. Parmar, I. D. Gaudet, J. R. Branch, D. H. Pike, V. Nanda, et al. Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir. 30(37):11204–11211, 2014.
Fujita, H., T. Nedachi, and M. Kanzaki. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp. Cell Res. 313(9):1853–1865, 2007.
Fujita, H., K. Shimizu, and E. Nagamori. Novel method for fabrication of skeletal muscle construct from the C2C12 myoblast cell line using serum-free medium AIM-V. Biotechnol. Bioeng. 103(5):1034–1041, 2009.
Garcia-Lizarribar, A., X. Fernandez-Garibay, F. Velasco-Mallorqui, A. G. Castano, J. Samitier, and J. Ramon-Azcon. Composite biomaterials as long-lasting scaffolds for 3D bioprinting of highly aligned muscle tissue. Macromol. Biosci.18:e1800167, 2018.
Gaudet, I. D., and D. I. Shreiber. Characterization of methacrylated type-I collagen as a dynamic, photoactive hydrogel. Biointerphases. 7(1–4):25, 2012.
Gong, H. Y., J. Park, W. Kim, J. Kim, J. Y. Lee, and W. G. Koh. A novel conductive and micropatterned PEG-based hydrogel enabling the topographical and electrical stimulation of myoblasts. ACS Appl. Mater. Interfaces. 11:47695–47706, 2019.
Gribova, V., C. Y. Liu, A. Nishiguchi, M. Matsusaki, T. Boudou, C. Picart, et al. Construction and myogenic differentiation of 3D myoblast tissues fabricated by fibronectin–gelatin nanofilm coating. Biochem. Biophys. Res. Commun. 474:515–521, 2016.
Hill, E., T. Boontheekul, and D. J. Mooney. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 12:1295–1304, 2006.
Hitti, M. Report: Nearly 5.6 Million Americans Paralyzed: Web MD, 2009.
Iyer, S. R., N. Udpa, and Y. Gao. Chitosan selectively promotes adhesion of myoblasts over fibroblasts. J. Biomed. Mater. Res. A. 103:1899–1906, 2015.
Jo, H., M. Sim, S. Kim, S. Yang, Y. Yoo, J. H. Park, et al. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 48:100–109, 2017.
Joglekar, D., R. Warren, D. Browe, E. Ekwueme, M. Dariani, N. D. Padliya, et al. Investigating the effects of fertilized egg yolk extract on myoblast proliferation and differentiation. Regen. Eng. Transl. Med. 6:125–137, 2020.
Kim, W., C. H. Jang, and G. H. Kim. A myoblast-laden collagen bioink with fully aligned Au nanowires for muscle-tissue regeneration. Nano Lett. 19:8612–8620, 2019.
Kim, W., H. Lee, J. Lee, A. Atala, J. J. Yoo, S. J. Lee, et al. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials.230:119632, 2020.
Ko, U. H., S. Park, H. Bang, M. Kim, H. Shin, and J. H. Shin. Promotion of myogenic maturation by timely application of electric field along the topographical alignment. Tissue Eng. A. 24:752–760, 2018.
Kung, F. H., D. Sillitti, A. B. Shrirao, D. I. Shreiber, and B. L. Firestein. Collagen nanofibre anisotropy induces myotube differentiation and acetylcholine receptor clustering. J. Tissue Eng. Regen. Med. 12:e2010–e2019, 2018.
Lee, H., W. Kim, J. Lee, J. J. Yoo, G. H. Kim, and S. J. Lee. Effect of hierarchical scaffold consisting of aligned dECM nanofibers and poly(lactide-co-glycolide) struts on the orientation and maturation of human muscle progenitor cells. ACS Appl. Mater. Interfaces. 11:39449–39458, 2019.
Levett, P. A., F. P. W. Melchels, K. Schrobback, D. W. Hutmacher, J. Malda, and T. J. Klein. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 10(1):214–223, 2014. https://doi.org/10.1016/j.actbio.2013.10.005.
Li, R., N. L. McRae, D. R. McCulloch, M. Boyd-Moss, C. J. Barrow, D. R. Nisbet, et al. Large and small assembly: combining functional macromolecules with small peptides to control the morphology of skeletal muscle progenitor cells. Biomacromolecules. 19:825–837, 2018.
Liao, I. C., J. B. Liu, N. Bursac, and K. W. Leong, editors. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. In: Annual Meeting of the Biomedical-Engineering-Society, 2 Oct 2008, St Louis, MO, 2008.
Liu, X., Y. Gao, X. Long, T. Hayashi, K. Mizuno, S. Hattori, et al. Type I collagen promotes the migration and myogenic differentiation of C2C12 myoblasts via the release of interleukin-6 mediated by FAK/NF-κB p65 activation. Food Funct. 11(1):328–338, 2020.
Lumia, R., and M. Shahinpoor. IPMC microgripper research and development. J. Phys. Conf. Ser. 127(1):1–15, 2008.
Manchineella, S., G. Thrivikraman, K. K. Khanum, P. C. Ramamurthy, B. Basu, and T. Govindaraju. Pigmented silk nanofibrous composite for skeletal muscle tissue engineering. Adv. Healthc. Mater. 5:1222–1232, 2016.
Markert, C. D., A. Atala, J. K. Cann, G. Christ, M. Furth, F. Ambrosio, et al. Mesenchymal stem cells: emerging therapy for Duchenne muscular dystrophy. PM&R. 1(6):547–559, 2009.
Mazzoccoli, J. P., D. L. Feke, H. Baskaran, and P. N. Pintauro. Mechanical and cell viability properties of crosslinked low- and high-molecular weight poly (ethylene glycol) diacrylate blends. J. Biomed. Mater. Res. A. 93(2):558–566, 2010.
Meriggioli, M. N., and D. B. Sanders. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 8(5):475–490, 2009.
Nagai, Y., H. Yokoi, K. Kaihara, and K. Naruse. The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials. 33:1044–1051, 2012.
Narayanan, N., Z. Jia, K. H. Kim, L. Kuang, P. Lengemann, G. Shafer, et al. Biomimetic glycosaminoglycan-based scaffolds improve skeletal muscle regeneration in a murine volumetric muscle loss model. Bioact. Mater. 6:1201–1213, 2021.
Narayanan, N., C. Jiang, C. Wang, G. Uzunalli, N. Whittern, D. Chen, et al. Harnessing fiber diameter-dependent effects of myoblasts toward biomimetic scaffold-based skeletal muscle regeneration. Front. Bioeng. Biotechnol. 8:203, 2020.
Neuhaus, R., N. Zahiri, J. Petrs, et al. Integrating ionic electroactive polymer actuators and sensors into adaptive building skins—potentials and limitations. Front. Built Environ. 2020. https://doi.org/10.3389/fbuil.2020.00095.
Ngan, C., A. Quigley, C. O’Connell, M. Kita, J. Bourke, G. G. Wallace, et al. 3D bioprinting and differentiation of primary skeletal muscle progenitor cells. Methods Mol. Biol. 2140:229–242, 2020.
Ostrovidov, S., S. Ahadian, J. Ramon-Azcon, V. Hosseini, T. Fujie, S. P. Parthiban, et al. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 11:582–595, 2017.
Ostrovidov, S., X. Shi, L. Zhang, X. Liang, S. B. Kim, T. Fujie, et al. Myotube formation on gelatin nanofibers–multi-walled carbon nanotubes hybrid scaffolds. Biomaterials. 35:6268–6277, 2014.
Pankongadisak, P., E. Tsekoura, O. Suwantong, and H. Uludag. Electrospun gelatin matrices with bioactive pDNA polyplexes. Int. J. Biol. Macromol. 149:296–308, 2020.
Park, J., J. H. Choi, S. Kim, I. Jang, S. Jeong, and J. Y. Lee. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 97:141–153, 2019.
Patel, K. H., A. J. Dunn, M. Talovic, G. J. Haas, M. Marcinczyk, H. Elmashhady, et al. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater.14:035010, 2019.
Patel, A., S. Vendrell-Gonzalez, G. Haas, M. Marcinczyk, N. Ziemkiewicz, M. Talovic, et al. Regulation of myogenic activity by substrate and electrical stimulation in vitro. BioResearch Open Access. 8:129–138, 2019.
Pollot, B. E., C. R. Rathbone, J. C. Wenke, and T. Guda. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. B. 106:672–679, 2018.
Porzionato, A., M. M. Sfriso, A. Pontini, V. Macchi, L. Petrelli, P. G. Pavan, et al. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 16:14808–14831, 2015.
Pruller, J., I. Mannhardt, T. Eschenhagen, P. S. Zammit, and N. Figeac. Satellite cells delivered in their niche efficiently generate functional myotubes in three-dimensional cell culture. PLoS ONE.13:e0202574, 2018.
Punga, A. R., and M. A. Ruegg. Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr. Opin. Pharmacol. 12(3):340–346, 2012.
Rando, T. A. Non-viral gene therapy for Duchenne muscular dystrophy: progress and challenges. Biochim. Biophys. Acta Mol. Basis Dis. 1772(2):263–271, 2007.
Romanazzo, S., G. Forte, M. Ebara, K. Uto, S. Pagliari, T. Aoyagi, et al. Substrate stiffness affects skeletal myoblast differentiation in vitro. Sci. Technol. Adv. Mater.13(6):064211, 2012.
Rowley, J. A., and D. J. Mooney. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 60:217–223, 2002.
Scott, T. E., A. Khalili, B. Newton, R. Warren, D. P. Browe, and J. W. Freeman. Characterization and optimization of a positively charged poly(ethylene glycol) diacrylate hydrogel as an actuating muscle tissue engineering scaffold. Polym. Adv. Technol. 30(10):2604–2612, 2019.
Serena, E., M. Flaibani, S. Carnio, L. Boldrin, L. Vitiello, P. De Coppi, et al. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol. Res. 30:207–214, 2008.
Somers, S. M., N. Y. Zhang, J. B. F. Morrissette-McAlmon, K. Tran, H. Q. Mao, and W. L. Grayson. Myoblast maturity on aligned microfiber bundles at the onset of strain application impacts myogenic outcomes. Acta Biomater. 94:232–242, 2019.
Ungerleide, J. L., T. D. Johnson, N. Rao, and K. L. Christman. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods. 84:53–59, 2015.
Ungerleider, J. L., T. D. Johnson, M. J. Hernandez, D. I. Elhag, R. L. Braden, M. Dzieciatkowska, et al. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. JACC Basic Transl. Sci. 1(1–2):32–44, 2016.
Vartanian, A. D., A. Audfray, B. A. Jaam, M. Janot, S. Legardinier, A. Maftah, et al. Protein O-fucosyltransferase 1 expression impacts myogenic C2C12 cell commitment via the Notch signaling pathway. Mol. Cell. Biol. 35(2):391–405, 2015.
Venugopal, J., L. L. Ma, T. Yong, and S. Ramakrishna. In vitro study of smooth muscle cells on polycaprolactone and collagen nanofibrous matrices. Cell Biol. Int. 29(10):861–867, 2005. https://doi.org/10.1016/j.cellbi.2005.03.026.
Villanueva, P., S. Pereira, A. Olmo, P. Perez, Y. Yuste, A. Yufera, et al. Electrical pulse stimulation of skeletal myoblasts cell cultures with simulated action potentials. J. Tissue Eng. Regen. Med. 13:1265–1269, 2019.
Wagner, K. R., N. Lechtzin, and D. P. Judge. Current treatment of adult Duchenne muscular dystrophy. Biochim. Biophys. Acta Mol. Basis Dis. 1772(2):229–237, 2007.
Wang, W., M. Fan, L. Zhang, S. H. Liu, L. Sun, and C. Y. Wang. Compatibility of hyaluronic acid hydrogel and skeletal muscle myoblasts. Biomed. Mater.4:025011, 2009.
Willmann, R., S. Possekel, J. Dubach-Powell, T. Meier, and M. A. Ruegg. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul. Disord. 19(4):241–249, 2009.
Yeo, M., and G. Kim. Nano/microscale topographically designed alginate/PCL scaffolds for inducing myoblast alignment and myogenic differentiation. Carbohydr. Polym.223:115041, 2019.
Yeo, M., and G. Kim. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 107:102–114, 2020.
Zhang, J., Z. Q. Hu, N. J. Turner, S. F. Teng, W. Y. Cheng, H. Y. Zhou, et al. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials. 89:114–126, 2016.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Elizabeth Cosgriff-Hernandez oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda Alarcón, Y.S., Jazwinska, D., Lymon, T. et al. The Use of Collagen Methacrylate in Actuating Polyethylene Glycol Diacrylate–Acrylic Acid Scaffolds for Muscle Regeneration. Ann Biomed Eng 51, 1165–1180 (2023). https://doi.org/10.1007/s10439-023-03139-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03139-8