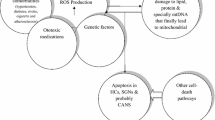
Abstract
Mitochondria are highly dynamic multifaceted organelles with various functions including cellular energy metabolism, reactive oxygen species (ROS) generation, calcium homeostasis, and apoptosis. Because of these diverse functions, mitochondria are key regulators of cell survival and death, and their dysfunction is implicated in numerous diseases, particularly neurodegenerative disorders such as Alzheimer’s Disease, Parkinson's Disease, and Huntington’s Disease. One of the most common neurodegenerative disorders is sensorineural hearing loss (SNHL). SNHL primarily originates from the degenerative changes in the cochlea, which is the auditory portion of the inner ear. Many cochlear cells contain an abundance of mitochondria and are metabolically highly active, rendering them susceptible to mitochondrial dysfunction. Indeed, the causal role of mitochondrial dysfunction in SNHL progression is well established, and therefore, targeted for treatment. In this review, we aim to compile the emerging findings in the literature indicating the role of mitochondrial dysfunction in the progression of sensorineural hearing loss and highlight potential therapeutics targeting mitochondrial dysfunction for hearing loss treatment.




Similar content being viewed by others
References
Afifi, P. O., and H. H. Elsanadiky. Audiological findings in children with ataxia-telangiectasia (A-T) syndrome. Int. J. Pediatr. Otorhinolaryngol. 92:94–98, 2017
Amati-Bonneau, P., et al. OPA1 R445H mutation in optic atrophy associated with sensorineural deafness. Ann. Neurol. 58:958–963, 2005
Bhatta, P., et al. Capsaicin protects against cisplatin ototoxicity by changing the STAT3/STAT1 ratio and activating cannabinoid (CB2) receptors in the cochlea. Sci. Rep. 2019. https://doi.org/10.1038/s41598-019-40425-9
Bielefeld, E. C., et al. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 127:914–919, 2007
Brown, K. D., et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20:1059–1068, 2014
Cabelof, D. C., et al. Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat. Res. 500:135–145, 2002
Campbell, K. C., et al. Oral D-methionine protects against cisplatin-induced hearing loss in humans: phase 2 randomized clinical trial in India. Int. J. Audiol. 2021. https://doi.org/10.1080/14992027.2021.1983215
Chen, H., A. Chomyn, and D. C. Chan. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280:26185–26192, 2005
Chen, B., et al. Increased mitochondrial DNA damage and decreased base excision repair in the auditory cortex of D-galactose-induced aging rats. Mol. Biol. Rep. 38:3635–3642, 2011
Cho, S. I., E.-R. Jo, and H. Song. Urolithin A attenuates auditory cell senescence by activating mitophagy. Sci. Rep. 12:7704, 2022
Chocron, E. S., E. Munkácsy, and A. M. Pickering. Cause or casualty: the role of mitochondrial DNA in aging and age-associated disease. Biochim. Biophys. Acta Mol. Basis Dis. 1865:285, 2019
Das, R., and O. Chakrabarti. Mitochondrial hyperfusion: a friend or a foe. Biochem. Soc. Trans. 48:631–644, 2020
De La Luz, M., and A. Sordo. Mitochondrial dysfunction and hearing loss. In: Handbook of Mitochondrial Dysfunction, edited by S. I. Ahmad. Boca Raton: CRC Press, 2019, pp. 163–170
Ding, E., et al. Analysis of polymorphisms associated with base excision repair in patients susceptible and resistant to noise-induced hearing loss. Dis. Mark. 2019. https://doi.org/10.1155/2019/9327106
Dong, T., et al. Opa1 prevents apoptosis and cisplatin-induced ototoxicity in murine cochleae. Front. Cell Dev. Biol. 2021. https://doi.org/10.3389/fcell.2021.744838
Doosti, A., et al. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health. 16:223–227, 2014
Fang, E. F., et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 157:882–896, 2014
Fang, E. F., et al. NAD + replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 24:566–581, 2016
Fang, E. F., et al. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 17:308, 2016
Fernandez-Marcos, P. J., and J. Auwerx. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93:884S, 2011
Fetoni, A. R., et al. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol. Ital. 29:70, 2009
Fetoni, A. R., R. Piacentini, A. Fiorita, G. Paludetti, and D. Troiani. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 1257:108–116, 2009
Finck, B. N., and D. P. Kelly. Peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 115:2540–2548, 2007
Fujimoto, C., and T. Yamasoba. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid. Med. Cell. Longev. 2014:1–6, 2014
Geisler, J. G. 2,4 Dinitrophenol as medicine. Cells. 8:280, 2019
Han, B., et al. Correlation between mitochondrial DNA 4977bp deletion and presbycusis: a system review and meta-analysis. Medicine (United States). 98:e16302, 2019
Hancock, J. T., R. Desikan, and S. J. Neill. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 29:345–350, 2001
He, Z., et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy. 13:1884–1904, 2017
Huang, T., R. Santarelli, and A. Starr. Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals. Brain Res. 1300:97–104, 2009
Intano, G. W., E. J. Cho, C. A. McMahan, and C. A. Walter. Age-related base excision repair activity in mouse brain and liver nuclear extracts. J. Gerontol. A. Biol. Sci. Med. Sci. 58:205–211, 2003
Kalinec, G. M., et al. Extracellular vesicles from auditory cells as nanocarriers for anti-inflammatory drugs and pro-resolving mediators. Front. Cell. Neurosci. 13:530, 2019
Kamogashira, T., C. Fujimoto, and T. Yamasoba. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed. Res. Int. 2015:1–7, 2015
Kamogashira, T., K. Hayashi, C. Fujimoto, S. Iwasaki, and T. Yamasoba. Functionally and morphologically damaged mitochondria observed in auditory cells under senescence-inducing stress. npj Aging Mech Dis. 2017. https://doi.org/10.1038/s41514-017-0002-2
Kauppila, J. H. K., et al. Base-excision repair deficiency alone or combined with increased oxidative stress does not increase mtDNA point mutations in mice. Nucleic Acids Res. 46:6642, 2018
Kil, J., C. Pierce, H. Tran, R. Gu, and E. D. Lynch. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 226:44–51, 2007
Kim, S. J., C. Park, J. N. Lee, and R. Park. Protective roles of fenofibrate against cisplatin-induced ototoxicity by the rescue of peroxisomal and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 353:43–54, 2018
Kim, J. H., et al. Protective effect of berberine chloride against cisplatin-induced ototoxicity. Genes Genomics. 44:1–7, 2022
Koide, Y., et al. Association between uncoupling protein 2 gene Ala55val polymorphism and sudden sensorineural hearing loss. J. Int. Adv. Otol. 14:166–169, 2018
Kopke, R., et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear. Res. 323:40–50, 2015
Kraus, F., and M. T. Ryan. The constriction and scission machineries involved in mitochondrial fission. J. Cell Sci. 130:2953–2960, 2017
Krokan, H. E., and M. Bjørås. Base excision repair. Cold Spring Harb. Perspect. Biol. 5:1–22, 2013
Lazarou, M., et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 524:309–314, 2015
Lee, Y., H. Y. Lee, R. A. Hanna, and A. B. Gustafsson. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 301:H1924, 2011
Lee, Y. K., H. G. Youn, H. J. Wang, and G. Yoon. Decreased mitochondrial OGG1 expression is linked to mitochondrial defects and delayed hepatoma cell growth. Mol. Cells. 35:489–497, 2013
Lin, H., et al. Inhibition of DRP-1-dependent mitophagy promotes cochlea hair cell senescence and exacerbates age-related hearing loss. Front. Cell. Neurosci. 13:550, 2019
Lisan, Q., et al. Prevalence of hearing loss and hearing aid use among adults in France in the CONSTANCES Study. JAMA Netw. Open. 5:e2217633–e2217633, 2022
Liu, Y.-H., et al. Involvement of the SIRT1/PGC-1α signaling pathway in noise-induced hidden hearing loss. Front. Physiol. 13:798395, 2022
Manche, S. K., M. Jangala, P. Putta, R. M. Koralla, and J. Akka. Association of oxidative stress gene polymorphisms with presbycusis. Gene. 593:277–283, 2016
Matsuda, N., et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189:211–221, 2010
Mokhtari, V., P. Afsharian, M. Shahhoseini, S. M. Kalantar, and A. Moini. A Review on various uses of N-acetyl cysteine. Cell J. 19:11–17, 2017
Moussa, Z., Z. M. A. Judeh, and S. A. Ahmed. Nonenzymatic exogenous and endogenous antioxidants. Free Radic. Med. Biol. 2019. https://doi.org/10.5772/INTECHOPEN.87778
Muderris, T., et al. Efficiency of resveratrol in the prevention and treatment of age-related hearing loss. Exp. Ther. Med. 2022. https://doi.org/10.3892/etm.2021.10962
Nascimento-dos-Santos, G., et al. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165686, 2020
Oh, J., C. K. Youn, Y. Jun, E. R. Jo, and S. I. Cho. Reduced mitophagy in the cochlea of aged C57BL/6J mice. Exp. Gerontol. 137:110946, 2020
Okur, M. N., et al. Cockayne syndrome proteins CSA and CSB maintain mitochondrial homeostasis through NAD+ signaling. Aging Cell. 2020. https://doi.org/10.1111/acel.13268
Okur, M. N., et al. Short-term NAD + supplementation prevents hearing loss in mouse models of Cockayne syndrome. npj Aging Mech Dis. 2020. https://doi.org/10.1038/s41514-019-0040-z
Okur, M. N., et al. TITLE: Long-term NAD+ supplementation prevents the progression of age-related hearing loss in mice. bioRxiv. 2022. https://doi.org/10.1101/2022.08.25.505332
Paciello, F., et al. Pioglitazone represents an effective therapeutic target in preventing oxidative/inflammatory cochlear damage induced by noise exposure. Front. Pharmacol. 2018. https://doi.org/10.3389/fphar.2018.01103
Perkins, G., et al. Altered outer hair cell mitochondrial and subsurface cisternae connectomics are candidate mechanisms for hearing loss in mice. J. Neurosci. 40:8556–8572, 2020
Pierelli, G., et al. Uncoupling protein 2: a key player and a potential therapeutic target in vascular diseases. Oxid. Med. Cell. Longev. 2017:1–11, 2017
Popov, L. D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 24:4892, 2020
Prakash, A., and S. Doublié. Base excision repair in the mitochondria. J. Cell. Biochem. 116:1490–1499, 2015
Puschner, B., and J. Schacht. Energy metabolism in cochlear outer hair cells in vitro. Hear. Res. 114:102–106, 1997
Salehi, P., et al. In silico transcriptomics identifies FDA-approved drugs and biological pathways for protection against cisplatin-induced hearing loss. bioRxiv. 2022. https://doi.org/10.1101/2022.01.26.477836
Scheibye-Knudsen, M., et al. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J. Exp. Med. 209:855–869, 2012
Schreiber, S. N., et al. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA. 101:6472–6477, 2004
Seidman, M., S. Babu, W. Tang, E. Naem, and W. S. Quirk. Effects of resveratrol on acoustic trauma. Otolaryngol. Head. Neck Surg. 129:463–470, 2003
Setz, C., et al. Induction of mitophagy in the HEI-OC1 auditory cell line and activation of the Atg12/LC3 pathway in the organ of Corti. Hear. Res. 361:52–65, 2018
Sharma, G., et al. Characterization of a novel variant in the HR1 domain of MFN2 in a patient with ataxia, optic atrophy and sensorineural hearing loss. bioRxiv. 2021. https://doi.org/10.1101/2021.01.11.426268
Shinomiya, H., et al. Hearing dysfunction in Xpa-deficient mice. Front. Aging Neurosci. 9:19, 2017
Tao, H., Y. Zhang, X. Zeng, G. I. Shulman, and S. Jin. Niclosamide ethanolamine–induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20:1263–1269, 2014
Tropitzsch, A., et al. Poly (ADP-Ribose) Polymerase-1 (PARP1) deficiency and pharmacological inhibition by pirenzepine protects from cisplatin-induced ototoxicity without affecting antitumor efficacy. Front. Cell. Neurosci. 2019. https://doi.org/10.3389/fncel.2019.00406
Ventura-Clapier, R., A. Garnier, and V. Veksler. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc. Res. 79:208–217, 2008
Virbasius, J. V., and R. C. Scarpulla. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA. 91:1309–1313, 1994
Vyssokikh, M. Y., et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc. Natl. Acad. Sci. USA. 117:6491–6501, 2020
Wang, Y., et al. Effects of D-methionine in mice with noise-induced hearing loss mice. J. Int. Med. Res. 47:3874, 2019
Weijian, Z., et al. PGC-1α overexpression promotes mitochondrial biogenesis to protect auditory cells against cisplatin-induced cytotoxicity. J. Bio-X Res. 2:81–86, 2019
Wilson, B. T., et al. The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care. Genet. Med. 18:483–493, 2015
Winterbourn, C. C., and M. B. Hampton. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45:549–561, 2008
Xiong, H., et al. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp. Gerontol. 51:8–14, 2014
Yamaguchi, T., M. Yoneyama, Y. Onaka, A. Imaizumi, and K. Ogita. Preventive effect of curcumin and its highly bioavailable preparation on hearing loss induced by single or repeated exposure to noise: a comparative and mechanistic study. J. Pharmacol. Sci. 134:225–233, 2017
Yang, Q., et al. PINK1 protects against gentamicin-induced sensory hair cell damage: possible relation to induction of autophagy and inhibition of p53 signal pathway. Front. Mol. Neurosci. 2018. https://doi.org/10.3389/fnmol.2018.00403
Youn, C. K., Y. Jun, E. R. Jo, and S. I. Cho. Age-related hearing loss in C57BL/6J Mice is associated with mitophagy impairment in the central auditory system. Int. J. Mol. Sci. 21:1–14, 2020
Yumusakhuylu, A. C., et al. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int. J. Pediatr. Otorhinolaryngol. 76:404–408, 2012
Zhang, L. N., et al. Novel small-molecule PGC-1α transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes. 62:1297–1307, 2013
Zhang, Y., et al. Decreased poly(ADP-ribose) polymerase 1 expression attenuates glucose oxidase-induced damage in rat cochlear marginal strial cells. Mol. Neurobiol. 53:5971–5984, 2016
Zhang, Y., et al. Increased mitophagy protects cochlear hair cells from aminoglycoside-induced damage. Autophagy. 2022. https://doi.org/10.1080/15548627.2022.2062872
Zhao, X. Y., et al. The effect of overexpression of PGC-1α on the mtDNA4834 common deletion in a rat cochlear marginal cell senescence model. Hear. Res. 296:13–24, 2013
Zhao, Z., et al. ROS-responsive nanoparticle as a berberine carrier for OHC-targeted therapy of noise-induced hearing loss. ACS Appl. Mater. Interfaces. 13:7102–7114, 2021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this review.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okur, M.N., Djalilian, H.R. Approaches to Mitigate Mitochondrial Dysfunction in Sensorineural Hearing Loss. Ann Biomed Eng 50, 1762–1770 (2022). https://doi.org/10.1007/s10439-022-03103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-03103-y