Abstract
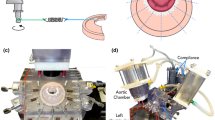
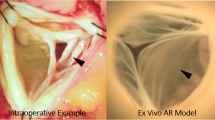
Systolic anterior motion (SAM) of the mitral valve (MV) is a complex pathological phenomenon often occurring as an iatrogenic effect of surgical and transcatheter intervention. While the aortomitral angle has long been linked to SAM, the mechanistic relationship is not well understood. We developed the first ex vivo heart simulator capable of recreating native aortomitral biomechanics, and to generate models of SAM, we performed anterior leaflet augmentation and sequential undersized annuloplasty procedures on porcine aortomitral junctions (n = 6). Hemodynamics and echocardiograms were recorded, and echocardiographic analysis revealed significantly reduced coaptation-septal distances confirming SAM (p = 0.003) and effective manipulation of the aortomitral angle (p < 0.001). Upon increasing the angle in our pathological models, we recorded significant increases (p < 0.05) in both coaptation-septal distance and multiple hemodynamic metrics, such as aortic peak flow and effective orifice area. These results indicate that an increased aortomitral angle is correlated with more efficient hemodynamic performance of the valvular system, presenting a potential, clinically translatable treatment opportunity for reducing the risk and adverse effects of SAM. As the standard of care shifts towards surgical and transcatheter interventions, it is increasingly important to better understand SAM biomechanics, and our advances represent a significant step towards that goal.








Similar content being viewed by others
Abbreviations
- SAM:
-
Systolic anterior motion
- MV:
-
Mitral valve
- LVOT:
-
Left ventricular outflow tract
- HCM:
-
Hypertrophic cardiomyopathy
- DOF:
-
Degree-of-freedom
- RMS:
-
Root mean square
- C-Sept:AMA:
-
Coaptation-septal distance to aortomitral angle ratio
References
Cape, E. G., D. Simons, A. Jimoh, A. E. Weyman, A. P. Yoganathan, and R. A. Levine. Chordal geometry determines the shape and extent of systolic anterior mitral motion: in vitro studies. J. Am. Coll. Cardiol. 13:1438–1448, 1989.
Guerrero, M., et al. Thirty-day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve-in-valve), failed surgical rings (valve-in-ring), and native valve with severe mitral annular calcification (valve-in-mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv.13:e008425, 2020.
Henry, W. L., C. E. Clark, J. M. Griffith, and S. E. Epstein. Mechanism of left ventricular outlfow obstruction in patients with obstructive asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis). Am. J. Cardiol. 35:337–345, 1975.
Ibrahim, M., C. Rao, H. Ashrafian, U. Chaudhry, A. Darzi, and T. Athanasiou. Modern management of systolic anterior motion of the mitral valve. Eur. J. Cardiothorac. Surg. 41:1260–1270, 2012.
Imbrie-Moore, A. M., M. H. Park, M. J. Paulsen, M. Sellke, R. Kulkami, H. Wang, Y. Zhu, J. M. Farry, A. T. Bourdillon, C. Callinan, H. J. Lucian, C. E. Hironaka, D. Deschamps, and Y. Joseph Woo. Biomimetic six-axis robots replicate human cardiac papillary muscle motion: pioneering the next generation of biomechanical heart simulator technology. J. R. Soc. Interface. 17:20200614, 2020.
Imbrie-Moore, A. M., M. J. Paulsen, Y. Zhu, H. Wang, H. J. Lucian, J. M. Farry, J. W. MacArthur, M. Ma, and Y. J. Woo. A novel cross-species model of Barlow’s disease to biomechanically analyze repair techniques in an ex vivo left heart simulator. J. Thorac. Cardiovasc. Surg. 161:1776–1783, 2021.
Jiang, L., R. A. Levine, M. E. King, and A. E. Weyman. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am. Heart J. 113:633–644, 1987.
Kelley, B. P., C. Gazda, J. A. Sivak, J. P. Vavalle, and T. T. Weickert. Successful mitraclip implantation in a barlow’s valve: a feasible alternative? CASE (Phila). 5:73–77, 2021.
Lefebvre, X. P., A. P. Yoganathan, and R. A. Levine. Insights from in-vitro flow visualization into the mechanism of systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy under steady flow conditions. J. Biomech. Eng. 114:406–413, 1992.
Lemke, R., and M. Kaltenbach. Systolic anterior movement of the mitral valve and the Venturi effect: an in vitro study. Z. Kardiol. 76(Suppl 3):87–90, 1987.
Levi, A., A. Sagie, and R. Kornowski. MitraClip-induced systolic anterior motion complicated by pericardial effusion: a case report. Catheter. Cardiovasc. Interv. 91:1371–1374, 2018.
Levine, R. A., G. J. Vlahakes, X. Lefebvre, J. L. Guerrero, E. G. Cape, A. P. Yoganathan, and A. E. Weyman. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation. 91:1189–1195, 1995.
Manabe, S., H. Kasegawa, T. Fukui, M. Tabata, T. Shimokawa, and S. Takanashi. Morphological analysis of systolic anterior motion after mitral valve repair. Interact. Cardiovasc. Thorac. Surg. 15:235–239, 2012.
Mihaileanu, S., J. P. Marino, S. Chauvaud, P. Perier, J. Forman, J. Vissoat, J. Julien, G. Dreyfus, P. Abastado, and A. Carpentier. Left ventricular outflow obstruction after mitral valve repair (Carpentier’s technique). Proposed mechanisms of disease. Circulation. 78:I78-84, 1988.
Park, C., Y. Fan, G. Hager, H. Yuk, M. Singh, A. Rojas, A. Hameed, M. Saeed, N. V. Vasilyev, T. W. J. Steele, X. Zhao, C. T. Nguyen, and E. T. Roche. An organosynthetic dynamic heart model with enhanced biomimicry guided by cardiac diffusion tensor imaging. Sci. Robot. 2020. https://doi.org/10.1126/scirobotics.aay9106.
Park, M. H., Y. Zhu, A. M. Imbrie-Moore, H. Wang, M. Marin-Cuartas, M. J. Paulsen, and Y. J. Woo. Heart valve biomechanics: the frontiers of modeling modalities and the expansive capabilities of ex vivo heart simulation. Front. Cardiovasc. Med.8:673689, 2021.
Paulsen, M. J., A. M. Imbrie-Moore, M. Baiocchi, H. Wang, C. E. Hironaka, H. J. Lucian, J. M. Farry, A. D. Thakore, Y. Zhu, M. Ma, J. W. MacArthur, and Y. J. Woo. Comprehensive ex vivo comparison of 5 clinically used conduit configurations for valve-sparing aortic root replacement using a 3-dimensional-printed heart simulator. Circulation. 142:1361–1373, 2020.
Raut, M., A. Maheshwari, and B. Swain. Awareness of “systolic anterior motion” in different conditions. Clin. Med. Insights Cardiol. 12:1179546817751921, 2018.
Schwammenthal, E., S. Nakatani, S. He, J. Hopmeyer, A. Sagie, A. E. Weyman, H. M. Lever, A. P. Yoganathan, J. D. Thomas, and R. A. Levine. Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: mismatch of posterior to anterior leaflet length and mobility. Circulation. 98:856–865, 1998.
Sherrid, M. V., C. K. Chu, E. Delia, A. Mogtader, and E. M. Dwyer. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 22:816–825, 1993.
Sherrid, M. V., D. Z. Gunsburg, S. Moldenhauer, and G. Pearle. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 36:1344–1354, 2000.
Varghese, R., S. Itagaki, A. C. Anyanwu, P. Trigo, G. Fischer, and D. H. Adams. Predicting systolic anterior motion after mitral valve reconstruction: using intraoperative transoesophageal echocardiography to identify those at greatest risk. Eur. J. Cardiothorac. Surg. 45:132–137, 2014.
Yoon, S.-H., et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 40:441–451, 2019.
Zhu, Y., A. M. Imbrie-Moore, M. J. Paulsen, B. Priromprintr, H. Wang, H. J. Lucian, J. M. Farry, and Y. J. Woo. Novel bicuspid aortic valve model with aortic regurgitation for hemodynamic status analysis using an ex vivo simulator. J. Thorac. Cardiovasc. Surg. 2020. https://doi.org/10.1016/j.jtcvs.2020.06.028.
Acknowledgments
This work was supported by the National Institutes of Health (NIH R01 HL152155, YJW), the National Science Foundation Graduate Research Fellowship Program (DGE-1656518, AMI), the Stanford Graduate Fellowship (AMI), and the Thoracic Surgery Foundation Resident Research Fellowship (YZ). We would also like to thank the generous donation by Donald and Sally O’Neal to support this research effort.
Funding
Dr. Y Joseph Woo: NIH R01 HL152155. Dr. Annabel M Imbrie-Moore: National Science Foundation, DGE-1656518.
Conflict of interest
The authors have nothing to disclose and report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 5383 KB)
Video 1 Operation of the aortomitral simulator. Each chamber interfaces with the respective heart anatomy to create a single, enclosed flow-loop driven by a pulsatile linear piston pump. (https://youtu.be/8ov0thE2gf0)
Supplementary file2 (MP4 5451 KB)
Video 2 Echocardiogram video demonstrating representative occurrence of systolic anterior motion in our ex vivo aortomitral simulator. (https://youtu.be/xU4XcknW8Zk)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, M.H., Imbrie-Moore, A.M., Zhu, Y. et al. The Critical Biomechanics of Aortomitral Angle and Systolic Anterior Motion: Engineering Native Ex Vivo Simulation. Ann Biomed Eng 51, 794–805 (2023). https://doi.org/10.1007/s10439-022-03091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-03091-z