Abstract
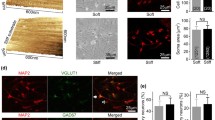
The stiffness of brain tissue changes during development and disease. These changes can affect neuronal morphology, specifically dendritic arborization. We previously reported that N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors regulate dendrite number and branching in a manner that is dependent on substrate stiffness. Since mitochondria affect the shape of dendrites, in this study, we determined whether the stiffness of substrates on which rat hippocampal neurons are grown affects mitochondrial characteristics and if glutamate receptors mediate the effects of substrate stiffness. Dendritic mitochondria are small, short, simple, and scarce in neurons cultured on substrates of 0.5 kPa stiffness. In contrast, dendritic mitochondria are large, long, complex, and low in number in neurons grown on substrates of 4 kPa stiffness. Dendritic mitochondria of neurons cultured on glass are high in number and small with complex shapes. Treatment of neurons grown on the stiffer gels or glass with the NMDA and AMPA receptor antagonists, 2-amino-5-phosphonopentanoic acid and 6-cyano-7-nitroquinoxaline-2,3-dione, respectively, results in mitochondrial characteristics of neurons grown on the softer substrate. These results suggest that glutamate receptors play important roles in regulating both mitochondrial morphology and dendritic arborization in response to substrate stiffness.







Similar content being viewed by others
References
Akashi, K., T. Kakizaki, H. Kamiya, M. Fukaya, M. Yamasaki, M. Abe, R. Natsume, M. Watanabe, and K. Sakimura. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci. 29:10869–10882, 2009
Bartolak-Suki, E., J. Imsirovic, Y. Nishibori, R. Krishnan, and B. Suki. Regulation of mitochondrial structure and dynamics by the cytoskeleton and mechanical factors. Int. J. Mol. Sci. 18:1812, 2017
Bernard-Trifilo, J. A., E. A. Kramar, R. Torp, C. Y. Lin, E. A. Pineda, G. Lynch, and C. M. Gall. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J. Neurochem. 93:834–849, 2005
Catanzaro, M. P., A. Weiner, A. Kaminaris, C. Li, F. Cai, F. Zhao, S. Kobayashi, T. Kobayashi, Y. Huang, H. Sesaki, and Q. Liang. Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy. FASEB J. 33:11096–11108, 2019
Chen, K., Y. Wang, X. Deng, L. Guo, and C. Wu. Extracellular matrix stiffness regulates mitochondrial dynamics through PINCH-1- and kindlin-2-mediated signalling. Curr. Res. Cell Biol.2:100008, 2021
Chihara, T., D. Luginbuhl, and L. Luo. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat. Neurosci. 10:828–837, 2007
Flanagan, L. A., Y. E. Ju, B. Marg, M. Osterfield, and P. A. Janmey. Neurite branching on deformable substrates. Neuroreport. 13:2411–2415, 2002
Fukumitsu, K., K. Fujishima, A. Yoshimura, Y. K. Wu, J. Heuser, and M. Kengaku. Synergistic action of dendritic mitochondria and creatine kinase maintains ATP homeostasis and actin dynamics in growing neuronal dendrites. J. Neurosci. 35:5707–5723, 2015
Galvao, J., B. Davis, M. Tilley, E. Normando, M. R. Duchen, and M. F. Cordeiro. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 28:1317–1330, 2014
Helle, S. C. J., Q. Feng, M. J. Aebersold, L. Hirt, R. R. Gruter, A. Vahid, A. Sirianni, S. Mostowy, J. G. Snedeker, A. Saric, T. Idema, T. Zambelli, and B. Kornmann. Mechanical force induces mitochondrial fission. Elife. 2017. https://doi.org/10.7554/eLife.30292
Jiang, F. X., B. Yurke, B. L. Firestein, and N. A. Langrana. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann. Biomed. Eng. 36:1565–1579, 2008
Juhasz, G., G. Vass, Z. Bozso, D. Budai, B. Penke, and V. Szegedi. Integrin activation modulates NMDA and AMPA receptor function of CA1 cells in a dose-related fashion in vivo. Brain Res. 1233:20–26, 2008
Kimura, T., and F. Murakami. Evidence that dendritic mitochondria negatively regulate dendritic branching in pyramidal neurons in the neocortex. J. Neurosci. 34:6938–6951, 2014
Kiryushko, D., V. Berezin, and E. Bock. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann. N. Y. Acad. Sci. 1014:140–154, 2004
Kramar, E. A., J. A. Bernard, C. M. Gall, and G. Lynch. Integrins modulate fast excitatory transmission at hippocampal synapses. J. Biol. Chem. 278:10722–10730, 2003
Langhammer, C. G., M. L. Previtera, E. S. Sweet, S. S. Sran, M. Chen, and B. L. Firestein. Automated Sholl analysis of digitized neuronal morphology at multiple scales: Whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A. 77:1160–1168, 2010
Lee, J. H., H. Y. Lee, and H. W. Kim. Adhesive proteins linked with focal adhesion kinase regulate neurite outgrowth of PC12 cells. Acta Biomater. 8:165–172, 2012
Lin, B., A. C. Arai, G. Lynch, and C. M. Gall. Integrins regulate NMDA receptor-mediated synaptic currents. J. Neurophysiol. 89:2874–2878, 2003
Lyra-Leite, D. M., A. P. Petersen, N. R. Ariyasinghe, N. Cho, and M. L. McCain. Mitochondrial architecture in cardiac myocytes depends on cell shape and matrix rigidity. J. Mol. Cell Cardiol. 150:32–43, 2021
Ma, L., J. X. Dong, W. R. Fu, X. Y. Li, J. Chen, and Y. Liu. Mitochondrial morphology and function impaired by dimethyl sulfoxide and dimethyl formamide. J. Bioenerg. Biomembr. 50:297–305, 2018
MacVicar, T., and T. Langer. Mechanometabolism: mitochondria promote resilience under pressure. Curr. Biol. 31:R859–R861, 2021
Previtera, M. L., and B. L. Firestein. Glutamate affects dendritic morphology of neurons grown on compliant substrates. Biotechnol. Prog. 31:1128–1132, 2015
Previtera, M. L., C. G. Langhammer, N. A. Langrana, and B. L. Firestein. Regulation of dendrite arborization by substrate stiffness is mediated by glutamate receptors. Ann. Biomed. Eng. 38:3733–3743, 2010
Qin, Y., W. Jiang, A. Li, M. Gao, H. Liu, Y. Gao, X. Tian, and G. Gong. The combination of paraformaldehyde and glutaraldehyde is a potential fixative for mitochondria. Biomolecules. 11(5):711, 2021
Richter, K. N., N. H. Revelo, K. J. Seitz, M. S. Helm, D. Sarkar, R. S. Saleeb, E. D’Este, J. Eberle, E. Wagner, C. Vogl, D. F. Lazaro, F. Richter, J. Coy-Vergara, G. Coceano, E. S. Boyden, R. R. Duncan, S. W. Hell, M. A. Lauterbach, S. E. Lehnart, T. Moser, T. F. Outeiro, P. Rehling, B. Schwappach, I. Testa, B. Zapiec, and S. O. Rizzoli. Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy. EMBO J. 37:139–159, 2018
Rintoul, G. L., A. J. Filiano, J. B. Brocard, G. J. Kress, and I. J. Reynolds. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 23:7881–7888, 2003
Sanchez, C., L. Ulloa, R. J. Montoro, J. Lopez-Barneo, and J. Avila. NMDA-glutamate receptors regulate phosphorylation of dendritic cytoskeletal proteins in the hippocampus. Brain Res. 765:141–148, 1997
Singh, P., S. Doshi, J. M. Spaethling, A. J. Hockenberry, T. P. Patel, D. M. Geddes-Klein, D. R. Lynch, and D. F. Meaney. N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. J. Biol. Chem. 287:4348–4359, 2012
Stukel, J. M., and R. K. Willits. Mechanotransduction of neural cells through cell-substrate interactions. Tissue Eng. Part B Rev. 22:173–182, 2016
Sur, S., C. J. Newcomb, M. J. Webber, and S. I. Stupp. Tuning supramolecular mechanics to guide neuron development. Biomaterials. 34:4749–4757, 2013
Swiatkowski, P., I. Nikolaeva, G. Kumar, A. Zucco, B. F. Akum, M. V. Patel, G. D’Arcangelo, and B. L. Firestein. Role of Akt-independent mTORC1 and GSK3beta signaling in sublethal NMDA-induced injury and the recovery of neuronal electrophysiology and survival. Sci. Rep. 7:1539, 2017
Tanaka, A., Y. Fujii, N. Kasai, T. Okajima, and H. Nakashima. Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment. PLoS ONE.13:e0191928, 2018
Tarus, D., L. Hamard, F. Caraguel, D. Wion, A. Szarpak-Jankowska, B. van der Sanden, and R. Auzely-Velty. Design of hyaluronic acid hydrogels to promote neurite outgrowth in three dimensions. ACS Appl. Mater. Interfaces. 8:25051–25059, 2016
Tharp, K. M., R. Higuchi-Sanabria, G. A. Timblin, B. Ford, C. Garzon-Coral, C. Schneider, J. M. Muncie, C. Stashko, J. R. Daniele, A. S. Moore, P. A. Frankino, S. Homentcovschi, S. S. Manoli, H. Shao, A. L. Richards, K. H. Chen, J. T. Hoeve, G. M. Ku, M. Hellerstein, D. K. Nomura, K. Saijo, J. Gestwicki, A. R. Dunn, N. J. Krogan, D. L. Swaney, A. Dillin, and V. M. Weaver. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 33:1322-1341.e1313, 2021
Wen, Y. Q., X. Gao, A. Wang, Y. Yang, S. Liu, Z. Yu, G. B. Song, and H. C. Zhao. Substrate stiffness affects neural network activity in an extracellular matrix proteins dependent manner. Colloids Surf. B Biointerfaces. 170:729–735, 2018
Westrate, L. M., J. A. Drocco, K. R. Martin, W. S. Hlavacek, and J. P. MacKeigan. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE.9:e95265, 2014
Wiemerslage, L., and D. Lee. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J. Neurosci. Methods. 262:56–65, 2016
Youle, R. J., and A. M. van der Bliek. Mitochondrial fission, fusion, and stress. Science. 337:1062–1065, 2012
Yu, Y., S. Liu, X. Wu, Z. Yu, Y. Xu, W. Zhao, I. Zavodnik, J. Zheng, C. Li, and H. Zhao. Mechanism of stiff substrates up-regulate cultured neuronal network activity. ACS Biomater. Sci. Eng. 5:3475–3482, 2019
Zhang, Q. Y., Y. Y. Zhang, J. Xie, C. X. Li, W. Y. Chen, B. L. Liu, X. A. Wu, S. N. Li, B. Huo, L. H. Jiang, and H. C. Zhao. Stiff substrates enhance cultured neuronal network activity. Sci. Rep. 4:6215, 2014
Acknowledgments
This work was supported by New Jersey Commission Brain Injury Research grants # CBIR14PIL005 (to N.N.B.) and CBIR14IRG019 (to B.L.F.), a Rutgers Division of Life Sciences Summer Undergraduate Research Fellowship (to S.K.), and a Rutgers Aresty Undergraduate Research Fellowship (to A.J.K.). A.O. was supported by New Jersey Commission Brain Injury Research fellowship # CBIR19FEL018 and National Institutes of Health Biotechnology Training Grant T32 GM008339-20.
Author information
Authors and Affiliations
Contributions
SK: Data curation; Formal analysis; Methodology; Visualization; Writing—original draft. AJK: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—review & editing; AO: Investigation; Methodology; NNB: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing—review & editing. BLF: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing—original draft; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Associate Editor Rebecca Willits oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumarapuram, S., Kunnath, A.J., Omelchenko, A. et al. Glutamate Receptors Mediate Changes to Dendritic Mitochondria in Neurons Grown on Stiff Substrates. Ann Biomed Eng 50, 1116–1133 (2022). https://doi.org/10.1007/s10439-022-02987-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-02987-0