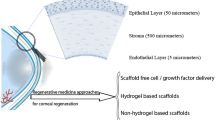
Abstract
3D bioprinting technology is a promising approach for corneal stromal tissue regeneration. In this study, gelatin methacrylate (GelMA) mixed with corneal stromal cells was used as a bioink. The visible light-based stereolithography (SLA) 3D bioprinting method was utilized to print the anatomically similar dome-shaped structure of the human corneal stroma. Two different concentrations of GelMA macromer (7.5 and 12.5%) were tested for corneal stroma bioprinting. Due to high macromer concentrations, 12.5% GelMA was stiffer than 7.5% GelMA, which made it easier to handle. In terms of water content and optical transmittance of the bioprinted scaffolds, we observed that scaffold with 12.5% GelMA concentration was closer to the native corneal stroma tissue. Subsequently, cell proliferation, gene and protein expression of human corneal stromal cells encapsulated in the bioprinted scaffolds were investigated. Cytocompatibility in 12.5% GelMA scaffolds was observed to be 81.86 and 156.11% at day 1 and 7, respectively, which were significantly higher than those in 7.5% GelMA scaffolds. Elongated corneal stromal cells were observed in 12.5% GelMA samples after 7 days, indicating the cell attachment, growth, and integration within the scaffold. The gene expression of collagen type I, lumican and keratan sulfate increased over time for the cells cultured in 12.5% GelMA scaffolds as compared to those cultured on the plastic tissue culture plate. The expression of collagen type I and lumican were also visualized using immunohistochemistry after 28 days. These findings imply that the SLA 3D bioprinting method with GelMA hydrogel bioinks is a promising approach for corneal stroma tissue biofabrication.
Graphical Abstract







Similar content being viewed by others
References
Arnalich-Montiel, F., J. L. Alió Del Barrio, and J. L. Alió. Corneal surgery in keratoconus: which type, which technique, which outcomes? Eye Vis. Lond. 3:2, 2016.
Arya, A. D., P. M. Hallur, A. G. Karkisaval, A. Gudipati, S. Rajendiran, V. Dhavale, B. Ramachandran, A. Jayaprakash, N. Gundiah, and A. Chaubey. Gelatin methacrylate hydrogels as biomimetic three-dimensional matrixes for modeling breast cancer invasion and chemoresponse in vitro. ACS Appl. Mater. Interfaces 8:22005–22017, 2016.
Bajaj, P., R. M. Schweller, A. Khademhosseini, J. L. West, and R. Bashir. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 16:247–276, 2014.
Baradaran-Rafii, A., M. Eslani, Z. Haq, E. Shirzadeh, M. J. Huvard, and A. R. Djalilian. Current and upcoming therapies for ocular surface chemical njuries. Ocul. Surf. 15:48–64, 2017.
Beems, E. M., and J. A. Van Best. Light transmission of the cornea in whole human eyes. Exp. Eye Res. 50:393–395, 1990.
Bulcke, A., B. Bogdanov, N. Rooze, E. Schacht, R. Cornelissen, and H. Berghmans. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1:31–38, 2000.
Celikkin, N., S. Mastrogiacomo, J. Jaroszewicz, X. Walboomers, and W. Swieszkowski. Gelatin methacrylate scaffold for bone tissue engineering: the influence of polymer concentration. J. Biomed. Mater. Res. Part A 106:201–209, 2017.
Claaßen, C., M. H. Claaßen, V. Truffault, L. Sewald, G. E. M. Tovar, K. Borchers, and A. Southan. Quantification of substitution of gelatin methacryloyl: best practice and current pitfalls. Biomacromolecules 19:42–52, 2018.
Dong, L., S.-J. Wang, X.-R. Zhao, Y.-F. Zhu, and J.-K. Yu. 3D-printed poly(e-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci. Rep. 7:1–9, 2017.
Dong, Z., Q. Yuan, K. Huang, W. Xu, G. Liu, and Z. Gu. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 9:17737–17744, 2019.
Duarte Campos, D. F., M. Rohde, M. Ross, P. Anvari, A. Blaeser, M. Vogt, C. Panfil, G. H. F. Yam, J. S. Mehta, H. Fischer, P. Walter, and M. Fuest. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 107:1945–1953, 2019.
Fagerholm, P., N. S. Lagali, K. Merrett, W. B. Jackson, R. Munger, Y. Liu, J. W. Polarek, M. Söderqvist, and M. Griffith. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2:46–61, 2010.
Fagerholm, P., N. S. Lagali, J. A. Ong, K. Merrett, W. B. Jackson, J. W. Polarek, E. J. Suuronen, Y. Liu, I. Brunette, and M. Griffith. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 35:2420–2427, 2014.
Fu, F., Z. Chen, Z. Zhao, H. Wang, L. Shang, Z. Gu, and Y. Zhao. Bio-inspired self-healing structural color hydrogel. Proc. Natl. Acad. Sci. USA 114:5900–5905, 2017.
Ghezzi, C. E., B. Marelli, F. G. Omenetto, J. L. Funderburgh, and D. L. Kaplan. Functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS ONE 12:1–18, 2017.
Gouveia, R. M., E. Koudouna, J. Jester, F. Figueiredo, and C. J. Connon. Template curvature influences cell alignment to create improved human corneal tissue equivalents. Adv. Biosyst. 1:1–10, Dec. 2017.
Gregor, A., E. Filová, M. Novák, J. Kronek, H. Chlup, M. Buzgo, V. Blahnová, V. Lukášová, M. Bartoš, A. Nečas, and J. Hošek. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 11:1–21, 2017.
Gungor-Ozkerim, P. S., I. Inci, Y. S. Zhang, A. Khademhosseini, and M. R. Dokmeci. Bioinks for 3D bioprinting: an overview. Biomater. Sci. 6:915–946, 2018.
Hacioglu, A., H. Yilmazer, and C. B. Ustundag. 3D printing for tissue engineering applications. J. Polytech. 21:221–227, 2018.
Hasan, A., A. Paul, N. E. Vrana, X. Zhao, A. Memic, Y.-S. Hwang, M. R. Dokmeci, and A. Khademhosseini. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 35:7308–7325, 2014.
Hashmani, K., M. J. Branch, L. E. Sidney, P. S. Dhillon, M. Verma, O. D. Mcintosh, A. Hopkinson, and H. S. Dua. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res. Ther. 4:1–13, 2013.
Ho, L. T. Y., A. M. Harris, H. Tanioka, N. Yagi, S. Kinoshita, B. Caterson, A. J. Quantock, R. D. Young, and K. M. Meek. A comparison of glycosaminoglycan distributions, keratan sulphate sulphation patterns and collagen fibril architecture from central to peripheral regions of the bovine cornea. Matrix Biol. 38:59–68, 2014.
Hwang, C. M., S. Sant, M. Masaeli, N. N. Kachouie, B. Zamanian, S.-H. Lee, and A. Khademhosseini. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2:35–43, 2010.
Isaacson, A., S. Swioklo, and C. J. Connon. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 173:188–193, 2018.
Kao, W. W.-Y., and C.-Y. Liu. Roles of lumican and keratocan on corneal transparency. Glycoconj. J. 19:275–285, 2002.
Kim, C., J. L. Young, A. W. Holle, K. Jeong, L. G. Major, J. H. Jeong, Z. M. Aman, D.-W. Han, Y. Hwang, J. P. Spatz, and Y. S. Choi. Stem cell mechanosensation on gelatin methacryloyl (GelMA) stiffness gradient hydrogels. Ann. Biomed. Eng. 48:893–902, 2020.
Koo, S., S. J. Ahn, H. Zhang, J. C. Wang, and E. K. F. Yim. Human corneal keratocyte response to micro-and nano-gratings on chitosan and PDMS. Cell. Mol. Bioeng. 4:399–410, 2011.
Kuete, V., O. Karaosmanoğlu, and H. Sivas. Chapter 10—anticancer activities of african medicinal spices and vegetables. In: Medical Spices and Vegetables from Africa, edited by A. Kuete. New York: Elsevier, 2017, pp. 271–297.
Lee, J.-H., and H.-W. Kim. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018. https://doi.org/10.1177/2041731418768285.
Lee, S.-J., W. Zhu, N. Castro, and L. G. Zhang. Biomaterials and 3D printing techniques for neural tissue regeneration. In: Neural Engineering: From Advanced Biomaterials to 3D Fabrication Techniques, edited by L. Zhang, and D. L. Kaplan. Geneva: Springer, 2016.
Li, F., D. Carlsson, C. Lohmann, E. Suuronen, S. Vascotto, K. Kobuch, H. Sheardown, R. Munger, M. Nakamura, and M. Griffith. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl. Acad. Sci. USA 100:15346–15351, 2003.
Liu, J., H. Zheng, P. S. P. Poh, H.-G. Machens, and A. F. Schilling. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 16:15997–16016, 2015.
Ludwig, P. E., T. J. Huff, and J. M. Zuniga. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J. Tissue Eng. 9:1–10, 2018.
Mahajan, S. D., W.-C. Law, R. Aalinkeel, J. Reynolds, B. B. Nair, K.-T. Yong, I. Roy, P. N. Prasad, and S. A. Schwartz. Chapter three—nanoparticle-mediated targeted delivery of antiretrovirals to the brain. In: Methods in Enzymology, edited by E. Düzgüneş. New York: Elsevier, 2012, pp. 41–60.
Massie, I., A. K. Kureshi, S. Schrader, A. J. Shortt, and J. T. Daniels. Optimization of optical and mechanical properties of real architecture for 3-dimensional tissue equivalents: towards treatment of limbal epithelial stem cell deficiency. Acta Biomater. 24:241–250, 2015.
McHale, M. K., N. M. Bergmann, and J. L. West. Chapter 38—histogenesis in three-dimensional scaffolds. In: Principles of Regenerative Medicine3rd, edited by A. Atala, R. Lanza, A. G. Mikos, and R. Nerem. Boston: Academic Press, 2019, pp. 661–674.
Merrett, K., P. Fagerholm, C. R. McLaughlin, S. Dravida, N. Lagali, N. Shinozaki, M. A. Watsky, R. Munger, Y. Kato, F. Li, C. J. Marmo, and M. Griffith. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: performance of type I versus type III collagen. Investig. Ophthalmol. Vis. Sci. 49:3887–3894, 2008.
Nagymihály, R., Z. Veréb, A. Facskó, M. C. Moe, and G. Petrovski. Effect of isolation technique and location on the phenotype of human corneal stroma-derived cells. Stem Cells Int. 2017. https://doi.org/10.1155/2017/9275248.
Nichol, J. W., S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar, and A. Khademhosseini. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31:5536–5544, 2010.
Nikkhah, M., M. Akbari, A. Paul, A. Memic, A. Dolatshahi-Pirouz, and A. Khademhosseini. Gelatin-based biomaterials for tissue engineering and stem cell bioengineering. In: Biomaterials from Nature for Advanced Devices and Therapies, edited by N. Nevesm, and R. Reis. Wiley: New York, 2016, pp. 37–62.
Patel, S., J. L. Alió, J. Javaloy, J. J. Perez-Santonja, A. Artola, and J. Rodriguez-Prats. Human cornea before and after refractive surgery using a new device: vCH-1. Cornea 27:1042–1049, 2008.
Patel, S., and L. Tutchenko. The refractive index of the human cornea: a review. Contact Lens Anterior Eye 42:575–580, 2019.
Pereira, R. F., and P. J. Bártolo. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015. https://doi.org/10.1002/app.42458.
Radaei, P., S. Mashayekhan, and S. Vakilian. Modeling and optimization of gelatin-chitosan micro-carriers preparation for soft tissue engineering: using response surface methodology. Mater. Sci. Eng. C 75:545–553, 2017.
Raman, R., and R. Bashir. Stereolithographic 3D bioprinting for biomedical applications. In: Essentials of 3D Biofabrication and Translation, edited by A. Atala, and J. J. Yoo. Cambridge: Academic Press, 2015, pp. 89–121.
Reinstein, D. Z., T. J. Archer, M. Gobbe, R. H. Silverman, and D. J. Coleman. Stromal thickness in the normal cornea: three-dimensional display with artemis very high-frequency digital ultrasound. J. Refract. Surg. 25:776–786, 2009.
Rizwan, M., G. S. L. Peh, H. P. Ang, N. C. Lwin, K. Adnan, J. S. Mehta, W. S. Tan, and E. K. F. Yim. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 120:139–154, 2017.
Rose, J. B., S. Pacelli, A. J. El Haj, H. S. Dua, A. Hopkinson, L. J. White, and F. R. A. J. Rose. Gelatin-based materials in ocular tissue engineering. Materials (Basel) 7:3106–3135, 2014.
Rothrauff, B. B., L. Coluccino, R. Gottardi, L. Ceseracciu, S. Scaglione, L. Goldoni, and R. S. Tuan. Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 12:159–170, 2018.
Ruberti, J., and J. Zieske. Prelude to corneal tissue engineering—Gaining control of collagen organization. Prog. Retin. Eye Res. 27:549–577, 2008.
Shankar, H., D. Taranath, C. Santhirathelagan, and K. Pesudovs. Anterior segment biometry with the Pentacam: comprehensive assessment of repeatability of automated measurements. J. Cataract. Refr. Surg. 34:103–113, 2008.
ShirzaeiSani, E., A. Kheirkhah, D. Rana, Z. Sun, W. Foulsham, A. Sheikhi, A. Khademhosseini, R. Dana, and N. Annabi. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 2019. https://doi.org/10.1126/sciadv.aav1281.
Sidney, L., L. E. Sidney, O. D. Mcintosh, and A. Hopkinson. Phenotypic change and induction of cytokeratin expression during in vitro culture of corneal stromal cells. IOVS 56:7225–7235, 2015.
Singh, M. R., S. Patel, and D. Singh. Chapter 9—natural polymer-based hydrogels as scaffolds for tissue engineering. In: Nanobiomaterials in Soft Tissue Engineering, edited by A. M. B. T. Grumezescu. Burlington: William Andrew Publishing, 2016, pp. 231–260.
Tan, Z., C. Parisi, L. Di Silvio, D. Dini, and A. E. Forte. Cryogenic 3D printing of super soft hydrogels. Sci. Rep. 7:1–11, 2017.
Taniguchi, D., K. Matsumoto, T. Tsuchiya, R. Machino, Y. Takeoka, A. Elgalad, K. Gunge, K. Takagi, T. Taura, G. Hatachi, N. Matsuo, N. Yamasaki, K. Nakayama, and T. Nagayasu. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Carsiovasc. Thorac. Surg. 26:745–752, 2018.
Tarassoli, S. P., Z. M. Jessop, A. Al-Sabah, N. Gao, S. Whitaker, S. Doak, and I. S. Whitaker. Skin tissue engineering using 3D bioprinting: an evolving research field. J. Plast. Reconstr. Aesthet. Surg. 71:615–623, 2018.
Vermeulen, N., G. Haddow, T. Seymour, A. Faulkner-Jones, and W. Shu. 3D bioprint me: a socioethical view of bioprinting human organs and tissues. J. Med. Ethics 43:618–624, 2017.
Wang, Z., R. Abdulla, B. Parker, R. Samanipour, S. Ghosh, and K. Kim. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015. https://doi.org/10.1088/1758-5090/7/4/045009.
Wang, D. Z., X. Jin, R. Dai, J. Holzman, and K. Kim. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 6:21099–21104, 2016.
Wang, Z., H. Kumar, Z. Tian, X. Jin, J. F. Holzman, F. Menard, and K. Kim. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl. Mater. Interfaces 10:26859–26869, 2018.
Wang, Z., Z. Tian, F. Menard, and K. Kim. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 9:44–101, 2017.
Wen, T., S. Xun, M. Haoye, S. Baichuan, C. Peng, L. Xuejian, Z. Kaihong, Y. Xuan, P. Jiang, and L. Shibi. 3D printed porous ceramic scaffolds for bone tissue engineering: a review. Biomater. Sci. 5:1690–1698, 2017.
Wilson, S. L., I. Wimpenny, M. Ahearne, S. Rauz, A. J. El Haj, and Y. Yang. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Funct. Mater. 22:3641–3649, 2012.
Wu, Z., X. Su, Y. Xu, B. Kong, W. Sun, and S. Mi. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 6:1–10, 2016.
Zhang, L., M. C. Anderson, and C.-Y. Liu. The role of corneal stroma: a potential nutritional source for the cornea. J. Nat. Sci. 3:e428, 2017.
Zhang, B., L. Gao, L. Ma, Y. Luo, H. Yang, and Z. Cui. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering 5:777–794, 2019.
Zhang, Y., Y.-C. Wang, O. Yuka, L. Zhangh, and C.-Y. Liu. Mouse corneal stroma fibroblast primary cell culture. Bio-Protoc. 2016. https://doi.org/10.21769/BioProtoc.1960.
Zhang, Y. S., K. Yue, J. Aleman, K. Mollazadeh-Moghaddam, S. M. Bakht, J. Yang, W. Jia, V. Dell’Erba, P. Assawes, S. R. Shin, M. R. Dokmeci, R. Oklu, and A. Khademhosseini. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 45:148–163, 2017.
Zhao, X., Q. Lang, L. Yildirimer, Z. Y. Lin, W. Cui, N. Annabi, K. W. Ng, M. R. Dokmeci, A. M. Ghaemmaghami, and A. Khademhosseini. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Heal. Mater. 5:108–118, 2016.
Zhao, X., W. Song, S. Liu, and L. Ren. Corneal regeneration by utilizing collagen based materials. Sci. China Chem. 59:1548–1553, 2016.
Zhu, J., and R. E. Marchant. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 8:607–626, 2011.
Acknowledgements
This project has received funding from the Sharif University of Technology under Grant Number of G960111, Ophthalmic Research Center of Shahid Beheshti University of Medical Sciences (IR.SBMU) under Grant Number 15848 and Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2014-04010). The authors also thank Dr. Elaheh Jooybar of the Sharif University of Technology, Kabilan Sakthivel and Mohamed Gamal of the University of British Columbia for their valuable suggestions and discussions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahdavi, S.S., Abdekhodaie, M.J., Kumar, H. et al. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann Biomed Eng 48, 1955–1970 (2020). https://doi.org/10.1007/s10439-020-02537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02537-6