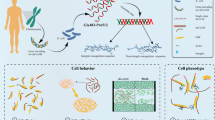
Abstract
A synthetic ‘chondroinductive’ biomaterial that could induce chondrogenesis without the need for growth factors, extracellular matrix, or pre-seeded cells could revolutionize orthopedic regenerative medicine. The objective of the current study was thus to introduce a synthetic SPPEPS peptide and evaluate its ability to induce chondrogenic differentiation. In the current study, dissolving a synthetic chondroinductive peptide candidate (100 ng/mL SPPEPS) in the culture medium of rat bone marrow-derived mesenchymal stem cells (rBMSCs) elevated collagen type II gene expression compared to the negative control (no growth factor or peptide in the cell culture medium) after 3 days. In addition, proteomic analyses indicated similarities in pathways and protein profiles between the positive control (10 ng/mL TGF-β3) and peptide group (100 ng/mL SPPEPS), affirming the potential of the peptide for chondroinductivity. Incorporating the SPPEPS peptide in combination with the RGD peptide in pentenoate-functionalized hyaluronic acid (PHA) hydrogels elevated the collagen type II gene expression of the rBMSCs cultured on top of the hydrogels compared to using either peptide alone. The evidence suggests that SPPEPS may be a chondroinductive peptide, which may be enhanced in combination with an adhesion peptide.








Similar content being viewed by others
References
Ables, J. L., J. J. Breunig, A. J. Eisch, and P. Rakic. Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 12:269–283, 2011. https://doi.org/10.1038/nrn3024.
Barry, F., R. E. Boynton, B. Liu, and J. M. Murphy. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 268:189–200, 2001. https://doi.org/10.1006/excr.2001.5278.
Burdick, J. A., R. L. Mauck, J. H. Gorman, and R. C. Gorman. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci. Transl. Med. 5:176ps174, 2013. https://doi.org/10.1126/scitranslmed.3003997.
Caprini, A., et al. A novel bioactive peptide: assessing its activity over murine neural stem cells and its potential for neural tissue engineering. N Biotechnol. 30:552–562, 2013. https://doi.org/10.1016/j.nbt.2013.03.005.
Ceylan, H., A. B. Tekinay, and M. O. Guler. Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface. Biomaterials 32:8797–8805, 2011. https://doi.org/10.1016/j.biomaterials.2011.08.018.
French, M. M., S. Rose, J. Canseco, and K. A. Athanasiou. Chondrogenic differentiation of adult dermal fibroblasts. Ann. Biomed. Eng. 32:50–56, 2004.
Gray, B. P., and K. C. Brown. Combinatorial peptide libraries: mining for cell-binding peptides. Chem. Rev. 114:1020–1081, 2014. https://doi.org/10.1021/cr400166n.
Itoh, S., et al. GSK-3alpha and GSK-3beta proteins are involved in early stages of chondrocyte differentiation with functional redundancy through RelA protein phosphorylation. j. Biol. Chem. 287:29227–29236, 2012. https://doi.org/10.1074/jbc.M112.372086.
Kiyotake, E. A., E. C. Beck, and M. S. Detamore. Cartilage extracellular matrix as a biomaterial for cartilage regeneration. Ann. N. Y. Acad. Sci. 1383:139–159, 2016. https://doi.org/10.1111/nyas.13278.
Kiyotake, E. A., A. W. Douglas, E. E. Thomas, S. L. Nimmo, and M. S. Detamore. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019. https://doi.org/10.1016/j.actbio.2019.01.041.
Li, A., Y. Wei, C. Hung, and G. Vunjak-Novakovic. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials 173:47–57, 2018. https://doi.org/10.1016/j.biomaterials.2018.05.004.
Li, Y., et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 80:423–430, 1995.
Lin, X., et al. Augmentation of osseous phenotypes in vivo with a synthetic peptide. J Orthop. Res. 25:531–539, 2007. https://doi.org/10.1002/jor.20303.
Lin, X. H., et al. B2A peptide induces chondrogenic differentiation in vitro and enhances cartilage repair in rats. J. Orthop. Res. 30:1221–1228, 2012. https://doi.org/10.1002/jor.22078.
Liu, S. Q., et al. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 31:7298–7307, 2010. https://doi.org/10.1016/j.biomaterials.2010.06.001.
Ludbrook, S. B., S. T. Barry, C. J. Delves, and C. M. Horgan. The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem. J. 369:311–318, 2003. https://doi.org/10.1042/BJ20020809.
Mahzoon, S., and M. S. Detamore. Peptides: drawing inspiration from cell–matrix interactions. Tissue Eng. B Rev. 2018. https://doi.org/10.1089/ten.teb.2018.0003.
Mahzoon, S., T. J. Siahaan, and M. S. Detamore. Bio-Instructive Scaffolds for Musculoskeletal Interfaces. In: Bio-instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine, edited by J. Brown, S. Kumbar, and B. Banik. Amsterdam: Elsevier, 2016.
McAllister, T. N., N. Dusserre, M. Maruszewski, and N. L’Heureux. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen. Med. 3:925–937, 2008. https://doi.org/10.2217/17460751.3.6.925.
Mu, D., et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 157:493–507, 2002. https://doi.org/10.1083/jcb.200109100.
Mummery, C. L., R. P. Davis, and J. E. Krieger. Challenges in using stem cells for cardiac repair. Sci. Transl. Med. 2:27ps17, 2010. https://doi.org/10.1126/scitranslmed.3000558.
Munger, J. S., J. G. Harpel, F. G. Giancotti, and D. B. Rifkin. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol. Biol. Cell 9:2627–2638, 1998.
Munger, J. S., et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328, 1999.
Mwale, F., et al. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J. Cell. Biochem. 88:1202–1213, 2003. https://doi.org/10.1002/jcb.10479.
Mwale, F., et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res. Ther. 13:R120, 2011. https://doi.org/10.1186/ar3423.
Renner, J. N., Y. Kim, and J. C. Liu. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. Pt A 18:2581–2589, 2012. https://doi.org/10.1089/ten.tea.2011.0400.
Roberts, A. B., et al. Mesoderm induction in Xenopus laevis distinguishes between the various TGF-beta isoforms. Growth Factors 3:277–286, 1990.
Shukla, A., et al. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat. Cell Biol. 11:777–784, 2009. https://doi.org/10.1038/ncb1885.
Svensen, N., J. G. Walton, and M. Bradley. Peptides for cell-selective drug delivery. Trends Pharmacol. Sci. 33:186–192, 2012. https://doi.org/10.1016/j.tips.2012.02.002.
Tamamura, Y., et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 280:19185–19195, 2005. https://doi.org/10.1074/jbc.M414275200.
Townsend, J. M., et al. Superior calvarial bone regeneration using pentenoate-functionalized hyaluronic acid hydrogels with devitalized tendon particles. Acta Biomater. 71:148–155, 2018. https://doi.org/10.1016/j.actbio.2018.02.013.
Verrecchio, A., et al. Design of peptides with high affinities for heparin and endothelial cell proteoglycans. J. Biol. Chem. 275:7701–7707, 2000. https://doi.org/10.1074/Jbc.275.11.7701.
Wei, Y., Y. Ji, L. Xiao, Q. Lin, and J. Ji. Different complex surfaces of polyethyleneglycol (PEG) and REDV ligand to enhance the endothelial cells selectivity over smooth muscle cells. Colloids surf. B 84:369–378, 2011. https://doi.org/10.1016/j.colsurfb.2011.01.028.
Wei, Y., et al. Surface engineering of cardiovascular stent with endothelial cell selectivity for in vivo re-endothelialisation. Biomaterials 34:2588–2599, 2013. https://doi.org/10.1016/j.biomaterials.2012.12.036.
Wojtowicz, A. M., et al. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31:2574–2582, 2010. https://doi.org/10.1016/j.biomaterials.2009.12.008.
Wu, D., and W. Pan. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 35:161–168, 2010. https://doi.org/10.1016/j.tibs.2009.10.002.
Xiao, Y., E. A. Friis, S. H. Gehrke, and M. S. Detamore. Mechanical testing of hydrogels in cartilage tissue engineering: beyond the compressive modulus. Tissue Eng. Part B Rev. 19:403–412, 2013. https://doi.org/10.1089/ten.TEB.2012.0461.
Xu, H. G., and W. Chen. Expression and significance of ENPP1, TNAP and ANK proteins in the degeneration of endplate chondrocytes in rats. Zhonghua Yi Xue Za Zhi 91:189–192, 2011.
Acknowledgments
The authors would like to thank Emi Kiyotake for assistance with preparing the manuscript. The authors acknowledge Dr. Susan Nimmo at the University of Oklahoma Magnetic Resonance Facility for help with conducting NMR. The authors acknowledge a potential conflict of interest as the authors (S.M. and M.D.) are actively pursuing intellectual property protection of the SPPEPS peptide.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahzoon, S., Townsend, J.M., Lam, T.N. et al. Effects of a Bioactive SPPEPS Peptide on Chondrogenic Differentiation of Mesenchymal Stem Cells. Ann Biomed Eng 47, 2308–2321 (2019). https://doi.org/10.1007/s10439-019-02306-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02306-0