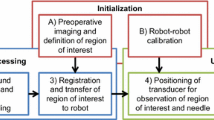
Abstract
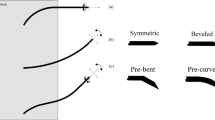
Intra-operative imaging is sometimes available to assist needle biopsy, but typical open-loop insertion does not account for unmodeled needle deflection or target shift. Closed-loop image-guided compensation for deviation from an initial straight-line trajectory through rotational control of an asymmetric tip can reduce targeting error. Incorporating robotic closed-loop control often reduces physician interaction with the patient, but by pairing closed-loop trajectory compensation with hands-on cooperatively controlled insertion, a physician’s control of the procedure can be maintained while incorporating benefits of robotic accuracy. A series of needle insertions were performed with a typical 18G needle using closed-loop active compensation under both fully autonomous and user-directed cooperative control. We demonstrated equivalent improvement in accuracy while maintaining physician-in-the-loop control with no statistically significant difference (p > 0.05) in the targeting accuracy between any pair of autonomous or individual cooperative sets, with average targeting accuracy of 3.56 mmrms. With cooperatively controlled insertions and target shift between 1 and 10 mm introduced upon needle contact, the system was able to effectively compensate up to the point where error approached a maximum curvature governed by bending mechanics. These results show closed-loop active compensation can enhance targeting accuracy, and that the improvement can be maintained under user directed cooperative insertion.








Similar content being viewed by others
References
Abayazid, M., R. J. Roesthuis, R. Reilink, and S. Misra. Integrating deflection models and image feedback for real-time flexible needle steering. IEEE Trans. Rob. 29(2):542–553, 2013.
Ahn, B., J. Kim, L. Ian, K. Rha, and H. Kim. Mechanical property characterization of prostate cancer using minimally motorized indenter in an ex vivo indentation experiment. Urology 76(4):1007–1011, 2010.
Bettini, A., S. Land, A. Okamura, and G. Hager. Vision assisted control for manipulation using virtual fixtures. IEEE Trans. Rob. 20(6):953–966, 2004.
Elayaperumal, S., J. H. Bae, D. Christensen, M. R. Cutkosky, B. L. Daniel, R. J. Black, J. M. Costa, F. Faridian, and B. Moslehi. MR-compatible biopsy needle with enhanced tip force sensing. IEEE World Haptics Conference, pp. 109–114, 2013.
Elayaperumal, S., J. H. Bae, B. L. Daniel, M. R. Cutkosky. Detection of membrane puncture with haptic feedback using a tip-force sensing needle. IEEE International Conference on Intelligent Robots and Systems, pp. 3975–3981, 2014.
Engh, J. A., D. S. Minhas, D. Kondziolka, and C. N. Riviere. Percutaneous intercerebral navigation by duty-cycled spinning of flexible bevel tipped needles. Neurosurgery 67(4):1117–1123, 2010.
Eslami, S., W. Shang, G. Li, N. Patel, G. S. Fischer, J. Tokuda, N. Hata, C. M. Tempany, and I. Iordachita. In-bore prostate transperineal interventions with an MRI-guided parallel manipulator: system development and preliminary evaluation. Int. J. Med. Robot. Comput. Assist. Surg. 12(2):98–109, 2015.
Farneback, G. Two-frame motion estimation based on polynomial expansion. In: Image Analysis, edited by J. Bigun, and T. Gustavsson. Heidelberg: Springer, 2003, pp. 363–370.
Hoyt, K., B. Casteneda, M. Zhang, P. Nigwekar, P. A. di Sant’Agnese, J. V. Joseph, J. Strang, D. Rubens, and J. Parker. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark. 4(4–5):213–225, 2001.
Hungr, N., J. Long, V. Beix, and J. Troccaz. A realistic deformable prostate phantom for multimodal imaging and needle-insertion procedures. Med. Phys. 39(4):2031–2041, 2012.
Jacopec, M., S. J. Harris, F. Rodrigues y Baena, P. Gomes, J. Cobb, and B. Davies. The first clinical application of a hands-on robotic knee surgery system. Comput. Aided Surg. 6:329–339, 2001.
Li, G. Robotic System Development for Precision MRI-Guided Needle-Based Interventions. PhD Dissertation, Department of Mechanical Engineering, June 2016.
Megali, G., O. Tonet, C. Stefanini, M. Boccadoro, V. Papaspyropoulos, L. Angelini, and P. Dario. A computer-assisted robotic ultrasound-guided biopsy system for video-assisted surgery. Medical Image Computing and Computer-Assisted Intervention, (MICCAI), pp. 343–350, 2016.
Minhas, D. S., J. A. Engh, M. M. Fenske, and C. N. Riviere. Modeling of needle steering via duty-cycled spinning. IEEE International Conference on Engineering in Medicine and Biology Society (EMBC), pp. 2756–2759, 2017.
Nycz, C. J., R. Gondokaryono, P. Carvalho, N. Patel, M. Wartenberg, J. G. Pilitsis, and G. S. Fischer. Mechanical validation of an MRI compatible stereotactic neurosurgery robot in preparation for pre-clinical trials. International Conference on Intelligent Robots and Systems (IROS), 2017.
Okamura, A. M., C. Simone, and M. D. O’Leary. Force modeling for needle insertion into soft tissue. IEEE Trans. Biomed. Eng. 10(51):1707–1716, 2004.
Pacchierotti, C., M. Abayazid, S. Misra, and D. Prattichizzo. Teleoperation of steerable flexible needles by combining kinesthetic and vibratory feedback. IEEE Trans. Haptics 7(4):551–556, 2014.
Patel, N. A., T. van Katwijk, G. Li, P. Moreira, W. Shang, S. Misra, and G. S. Fischer. Closed-loop asymmetric-tip needle steering under continuous intraoperative MRI guidance. IEEE International Conference on Engineering in Medicine and Biology Society (EMBC), pp. 6687–6690, 2015.
Podder, T., D. Clark, J. Sherman, D. Fuller, E. Messing, D. Rubens, J. Strang, R. Brasacchio, L. Liao, W. Ng, and Y. Yu. In vivo motion and force measurement of surgical needle intervention during prostate brachytherapy. Med. Phys. 33(8):2915–2922, 2006.
Prasad, S. K., M. Kitagawa, G. S. Fischer, J. Zand, M. A. Talamini, R. H. Taylor, and A. M. Okamura. A modular 2-DOF force-sensing instrument for laproscopic surgery. Medical Image Computing and Computer Assisted Interventions Conference (MICCAI), pp. 279–286, 2013.
Rossa, C., N. Usmani, R. Sloboda, and M. Tavakoli. A hand-held assistant for semiautomated percutaneous needle steering. IEEE Trans. Biomed. Eng. 64(3):637–648, 2017.
Schneider, O., J. Troccaz, O. Chavanon, and D. Blin. PADyC: a synergistic robot for cardiac puncturing. IEEE International Conference on Robotics and Automation (ICRA), pp. 2883–2888, 2000.
Stoianovici, D., C. Kim, G. Srimathveeravalli, P. Sebrecht, D. Petrisor, J. Coleman, S. B. Solomon, and H. Hricak. MRI-safe robot for endorectal prostate biopsy. IEEE Trans. Mechatron. 19(4):1289–1299, 2014.
Swensen, J. P. and N. J. Cowan. Torsional dynamics compensation enhances robotic control of tip-steerable needles. IEEE International Conference on Robotics and Automation (ICRA), pp. 1601–1606, 2012.
Tokuda, J., G. S. Fischer, X. Papademetris, Z. Yaniv, L. Ibanex, P. Cheng, H. Liu, J. Blevins, J. Arata, A. J. Golby, T. Kapur, S. Pieper, E. C. Burdette, G. Fichtinger, C. M. Tempany, and N. Hata. OpenIGTLink: an open network protocol for image-guided therapy environment. International Journal of Medical Robotics 5(4):423–434, 2009.
Tokuda, J., K. Tuncali, G. Li, N. Patel, T. Heffter, G. S. Fischer, I. Iordachita, E. C. Burdette, N. Hata, and C. Tempany. In-Bore MRI-Guided transperineal prostate biopsy using 4-DOF needle-guide manipulator. International Society for Magnetic Resonance in Medicine, 2016.
Troccaz, J., M. Peshkin, and B. Davies. Guiding systems for computer aided surgery: introducing synergistic devise and discussing the different approaches. Med. Image Anal. 2(2):101–118, 1998.
Tse, Z. T. H., H. Elhawary, M. Rea, B. Davies, I. Young, and M. Lamperth. Haptic needle unit for MR-guided biopsy and its control. IEEE Trans. Mechatron. 17(1):183–187, 2012.
Vigaru, B., D. Petrisor, A. Patriciu, D. Mazilu, and D. Stoianovici. MR compatible actuation for medical instrumentation. IEEE International Conference on Automation, Quality and Testing, Robotics, pp. 49–52, 2008.
Vrooijink, G. J., M. Abayazid, S. Patil, R. Alterovitz, and S. Misra. Needle path planning and steering in a three-dimensional non-static environment using two-dimensional ultrasound images. Int. J. Robot. Res. 33(10):1361–1374, 2014.
Wartenberg, M., J. Schornak, P. Carvalho, N. Patel, I. Iordachita, C. Tempany, N. Hata, J. Tokuda, and G. S. Fischer. Closed-loop autonomous needle steering during cooperatively controlled needle insertions for MRI-guided pelvic interventions. The 10th Hamlyn Symposium on Medical Robotics, pp. 33–34, 2017.
Webster, J., J. S. Kim, N. J. Cowan, G. S. Chirikjian, and A. M. Okamura. Nonholonomic modeling of needle steering. Int. J. Robot. Res. 25(5–6):509–524, 2006.
Acknowledgments
This research was funded by NIH R01 CA111288, NIH R01 CA166379 and NIH R01 EB020667.
Disclosure
NH has a financial interest in Harmonus, a company developing Image Guided Therapy products. NH’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kevin Cleary oversaw the review of this article.
Marek Wartenberg and Joseph Schornak shared first authorship.
Rights and permissions
About this article
Cite this article
Wartenberg, M., Schornak, J., Gandomi, K. et al. Closed-Loop Active Compensation for Needle Deflection and Target Shift During Cooperatively Controlled Robotic Needle Insertion. Ann Biomed Eng 46, 1582–1594 (2018). https://doi.org/10.1007/s10439-018-2070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2070-2