Abstract
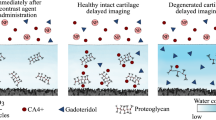
Impact injuries of cartilage may initiate post-traumatic degeneration, making early detection of injury imperative for timely surgical or pharmaceutical interventions. Cationic (positively-charged) CT contrast agents detect loss of cartilage proteoglycans (PGs) more sensitively than anionic (negatively-charged) or non-ionic (non-charged, i.e., electrically neutral) agents. However, degeneration related loss of PGs and increase in water content have opposite effects on the diffusion of the cationic agent, lowering its sensitivity. In contrast to cationic agents, diffusion of non-ionic agents is governed only by steric hindrance and water content of cartilage. We hypothesize that sensitivity of an iodine(I)-based cationic agent may be enhanced by simultaneous use of a non-ionic gadolinium(Gd)-based agent. We introduce a quantitative dual energy CT technique (QDECT) for simultaneous quantification of two contrast agents in cartilage. We employ this technique to improve the sensitivity of cationic CA4+ (q =+4) by normalizing its partition in cartilage with that of non-ionic gadoteridol. The technique was evaluated with measurements of contrast agent mixtures of known composition and human osteochondral samples (n = 57) after immersion (72 h) in mixture of CA4+ and gadoteridol. Samples were arthroscopically graded and biomechanically tested prior to QDECT (50/100 kV). QDECT determined contrast agent mixture compositions correlated with the true compositions (R2= 0.99, average error = 2.27%). Normalizing CA4+ partition in cartilage with that of gadoteridol improved correlation with equilibrium modulus (from ρ = 0.701 to 0.795). To conclude, QDECT enables simultaneous quantification of I and Gd contrast agents improving diagnosis of cartilage integrity and biomechanical status.




Similar content being viewed by others
References
Adair, G. S. On the Donnan equilibrium and the equations of Gibbs. Science 58:13, 1923.
Anderson, D. D., S. Chubinskaya, F. Guilak, J. A. Martin, T. R. Oegema, S. A. Olson, and J. A. Buckwalter. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J. Orthop. Res. 29:802–809, 2011.
Arkill, K. P., and C. P. Winlove. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthr. Cartil. 16:708–714, 2008.
Bansal, P. N., N. S. Joshi, V. Entezari, B. C. Malone, R. C. Stewart, B. D. Snyder, and M. W. Grinstaff. Cationic contrast agents improve quantification of glycosaminoglycan (GAG) content by contrast enhanced CT imaging of cartilage. J. Orthop. Res. 29:704–709, 2011.
Bansal, P. N., R. C. Stewart, V. Entezari, B. D. Snyder, and M. W. Grinstaff. Contrast agent electrostatic attraction rather than repulsion to glycosaminoglycans affords a greater contrast uptake ratio and improved quantitative CT imaging in cartilage. Osteoarthr. Cartil. 19:970–976, 2011.
Bay-Jensen, A.-C., S. Hoegh-Madsen, E. Dam, K. Henriksen, B. C. Sondergaard, P. Pastoureau, P. Qvist, and M. A. Karsdal. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol. Int. 30:435–442, 2010.
Berberat, J. E., M. J. Nissi, J. S. Jurvelin, and M. T. Nieminen. Assessment of interstitial water content of articular cartilage with T1 relaxation. Magn. Reson. Imaging 27:727–732, 2009.
Brittberg, M., and C. S. Winalski. Evaluation of cartilage injuries and repair. J. Bone Jt Surg. Am. 85-A(Suppl):58–69, 2003.
Brocklehurst, R., M. T. Bayliss, A. Maroudas, H. L. Coysh, M. A. Freeman, P. A. Revell, and S. Y. Ali. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J. Bone Jt Surg. Am. 66:95–106, 1984.
Buckwalter, J. A., H. J. Mankin, and A. J. Grodzinsky. Articular cartilage and osteoarthritis. Instr. Course Lect. 54:465–480, 2005.
Correa, D., and S. A. Lietman. Articular cartilage repair: current needs, methods and research directions. Semin. Cell Dev. Biol. 62:67–77, 2017.
Ewers, B. J., V. M. Jayaraman, R. F. Banglmaier, and R. C. Haut. Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. J. Biomech. 35:747–755, 2002.
Hayes, W. C., L. M. Keer, G. Herrmann, and L. F. Mockros. A mathematical analysis for indentation tests of articular cartilage. J. Biomech. 5:541–551, 1972.
Honkanen, J. T. J., M. J. Turunen, J. D. Freedman, S. Saarakkala, M. W. Grinstaff, J. H. Ylärinne, J. S. Jurvelin, and J. Töyräs. Cationic contrast agent diffusion differs between cartilage and meniscus. Ann. Biomed. Eng. 44:2913–2921, 2016.
Honkanen, J. T. J., M. J. Turunen, V. Tiitu, J. S. Jurvelin, and J. Töyräs. Transport of iodine is different in cartilage and meniscus. Ann. Biomed. Eng. 44:2114–2122, 2016.
Julkunen, P., R. K. Korhonen, W. Herzog, and J. S. Jurvelin. Uncertainties in indentation testing of articular cartilage: a fibril-reinforced poroviscoelastic study. Med. Eng. Phys. 30:506–515, 2008.
Kokkonen, H. T., J. S. Jurvelin, V. Tiitu, and J. Töyräs. Detection of mechanical injury of articular cartilage using contrast enhanced computed tomography. Osteoarthr. Cartil. 19:295–301, 2011.
Kokkonen, H. T., J.-S. Suomalainen, A. Joukainen, H. Kröger, J. Sirola, J. S. Jurvelin, J. Salo, and J. Töyräs. In vivo diagnostics of human knee cartilage lesions using delayed CBCT arthrography. J. Orthop. Res. 32:403–412, 2014.
Kokkonen, H. T., H. C. Chin, J. Töyräs, J. S. Jurvelin, and T. M. Quinn. Solute transport of negatively charged contrast agents across articular surface of injured cartilage. Ann. Biomed. Eng. 45:973–981, 2016.
Korhonen, R. K., M. S. Laasanen, J. Töyräs, R. Lappalainen, H. J. Helminen, and J. S. Jurvelin. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J. Biomech. 36:1373–1379, 2003.
Kulmala, K. A. M., H. M. Karjalainen, H. T. Kokkonen, V. Tiitu, V. Kovanen, M. J. Lammi, J. S. Jurvelin, R. K. Korhonen, and J. Töyräs. Diffusion of ionic and non-ionic contrast agents in articular cartilage with increased cross-linking—contribution of steric and electrostatic effects. Med. Eng. Phys. 35:1415–1420, 2013.
Lakin, B. A., H. Patel, C. Holland, J. D. Freedman, J. S. Shelofsky, B. D. Snyder, K. S. Stok, and M. W. Grinstaff. Contrast-enhanced CT using a cationic contrast agent enables non-destructive assessment of the biochemical and biomechanical properties of mouse tibial plateau cartilage. J. Orthop. Res. 34:1130–1138, 2016.
Lakin, B. A., B. D. Snyder, and M. W. Grinstaff. Assessing cartilage biomechanical properties: techniques for evaluating the functional performance of cartilage in health and disease. Annu. Rev. Biomed. Eng. 19:27–55, 2017.
Li, X., V. Pedoia, D. Kumar, J. Rivoire, C. Wyatt, D. Lansdown, K. Amano, N. Okazaki, D. Savic, M. F. Koff, J. Felmlee, S. L. Williams, and S. Majumdar. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthr. Cartil. 23:2214–2223, 2015.
Link, T. M., J. Neumann, and X. Li. Prestructural cartilage assessment using MRI. J. Magn. Reson. Imaging 45:949–965, 2017.
Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12:233–248, 1975.
Maroudas, A., P. Bullough, S. A. Swanson, and M. A. Freeman. The permeability of articular cartilage. J. Bone Jt Surg. Br. 50:166–177, 1968.
Muir, H., P. Bullough, and A. Maroudas. The distribution of collagen in human articular cartilage with some of its physiological implications. J. Bone Jt Surg. Br. 52:554–563, 1970.
Myller, K. A. H., M. J. Turunen, J. T. J. Honkanen, S. P. Vaananen, J. T. Iivarinen, J. Salo, J. S. Jurvelin, and J. Töyräs. In vivo contrast-enhanced cone beam CT provides quantitative information on articular cartilage and subchondral bone. Ann. Biomed. Eng. 45:811–818, 2017.
Pouran, B., V. Arbabi, A. A. Zadpoor, and H. Weinans. Isolated effects of external bath osmolality, solute concentration, and electrical charge on solute transport across articular cartilage. Med. Eng. Phys. 38:1399–1407, 2016.
Rangacharyulu, C. Physics of Nuclear Radiations Concepts, Techniques and Applications. Boca Raton: Taylor and Francis, 2013.
Saltybaeva, N., M. E. Jafari, M. Hupfer, and W. A. Kalender. Estimates of effective dose for CT scans of the lower extremities. Radiology 273:153–159, 2014.
Saukko, A. E. A., J. T. J. Honkanen, W. Xu, S. P. Vaananen, J. S. Jurvelin, V.-P. Lehto, and J. Töyräs. Dual contrast CT method enables diagnostics of cartilage injuries and degeneration using a single CT image. Ann. Biomed. Eng. 2017. https://doi.org/10.1007/s10439-017-1916-3.
Stewart, R. C., P. N. Bansal, V. Entezari, H. Lusic, R. M. Nazarian, B. D. Snyder, and M. W. Grinstaff. Contrast-enhanced CT with a high-affinity cationic contrast agent for imaging ex vivo bovine, intact ex vivo rabbit, and in vivo rabbit cartilage. Radiology 266:141–150, 2013.
Stewart, R. C., J. T. J. Honkanen, H. T. Kokkonen, V. Tiitu, S. Saarakkala, A. Joukainen, B. D. Snyder, J. S. Jurvelin, M. W. Grinstaff, and J. Töyräs. Contrast-enhanced computed tomography enables quantitative evaluation of tissue properties at intrajoint regions in cadaveric knee cartilage. Cartilage 8:391–399, 2017.
Stewart, R. C., A. N. Patwa, H. Lusic, J. D. Freedman, M. Wathier, B. D. Snyder, A. Guermazi, and M. W. Grinstaff. Synthesis and preclinical characterization of a cationic iodinated imaging contrast agent (CA4 +) and its use for quantitative computed tomography of ex vivo human hip cartilage. J. Med. Chem. 60:5543–5555, 2017.
Zou, G. Y. Toward using confidence intervals to compare correlations. Psychol. Methods 12:399–413, 2007.
Acknowledgments
Sandra Sefa (B.Sc.) is acknowledged for assistance with the biomechanical measurements. Jaakko Sarin, M.Sc.(Tech) is acknowledged for assistance in sample extraction. Academy of Finland (Projects 269315, 307932), Kuopio University Hospital (VTR 5041746, 5041757, PY210), Instrumentarium Science Foundation (170033) and Doctoral Program in Science, Technology and Computing (SCITECO, University of Eastern Finland) are acknowledged for financial support.
Conflicts of interests
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Bhattarai, A., Honkanen, J.T.J., Myller, K.A.H. et al. Quantitative Dual Contrast CT Technique for Evaluation of Articular Cartilage Properties. Ann Biomed Eng 46, 1038–1046 (2018). https://doi.org/10.1007/s10439-018-2013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2013-y