Abstract
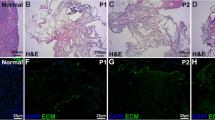
Since folliculogenesis requires a powerful cell–matrix interaction, natural scaffolds seem to be needed for follicular culture. Human amniotic membrane (HAM) offers promise as a support of in vitro ovarian follicular culture. HAM was decellularized with trypsin and EDTA. DNA and histology assays were performed to determine the elimination rate of genomic components. Cyto-biocompatibility of decellular AM (DAM) was verified by the cell viability (MTT) test. The small parts of intact amniotic membrane (IAM) and DAM were coated on the bottom of 96-well and each well was filled with 150 µL of base medium. Mouse primary-secondary (PS) follicles were separated to three groups: 1—culture in base medium (Control), 2—culture on IAM and 3—culture on DAM. Follicular size, morphology, viability, estradiol production and genes expression were evaluated and IAM group showed better growth and development in follicle culture. The viability rate and estradiol production in both experimental groups were statistically higher than the Control. Gdf9, Bmp15 and Cx37 were found to have higher expression levels in IAM group. Also, maximum apoptotic and survival indexes were determined in Control and IAM groups, respectively. Finally, IAM provides a better protective environment for mouse PS follicular culture that can reduce apoptosis level.








Similar content being viewed by others
References
Amorim, C. A., and A. Shikanov. The artificial ovary: current status and future perspectives. Future Oncol. 12(20):2323–2332, 2016.
Amorim, C. A., et al. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod. 24(1):92–99, 2009.
Annabi, N., et al. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 30(27):4550–4557, 2009.
Berkholtz, C. B., L. D. Shea, and T. K. Woodruff. Extracellular matrix functions in follicle maturation. Semin. Reprod. Med. 24(4):262–269, 2006.
Berkholtz, C. B., et al. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem. Cell Biol. 126(5):583–592, 2006.
Bley, M. A., P. E. Saragueta, and J. L. Baranao. Concerted stimulation of rat granulosa cell deoxyribonucleic acid synthesis by sex steroids and follicle-stimulating hormone. J. Steroid Biochem. Mol. Biol. 62(1):11–19, 1997.
Camboni, A., et al. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 67(1):64–69, 2013.
Carson, D. D., and J. P. Tang. Estrogen induces N-linked glycoprotein expression by immature mouse uterine epithelial cells. Biochemistry 28(20):8116–8123, 1989.
Chen, Y. J., et al. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials 33(2):455–463, 2012.
Gholipourmalekabadi, M., et al. Decellularized human amniotic membrane: how viable is it as a delivery system for human adipose tissue-derived stromal cells? Cell Prolif. 49(1):115–121, 2016.
Gilbert, T. W., T. L. Sellaro, and S. F. Badylak. Decellularization of tissues and organs. Biomaterials 27(19):3675–3683, 2006.
Green, D. M., et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J. Clin. Oncol. 27(14):2374–2381, 2009.
Guo, Q., et al. A new candidate substrate for cell-matrix adhesion study: the acellular human amniotic matrix. J. Biomed. Biotechnol. 2012:306083, 2012.
Hornick, J. E., et al. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod. 27(6):1801–1810, 2012.
Hornick, J. E., et al. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145(1):19–32, 2013.
Hsueh, A. J., et al. Intraovarian control of early folliculogenesis. Endocr. Rev. 36(1):1–24, 2015.
Jeruss, J. S., and T. K. Woodruff. Preservation of fertility in patients with cancer. N. Engl. J. Med. 360(9):902–911, 2009.
Koizumi, N. J., et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 20(3):173–177, 2000.
Kristensen, S. G., et al. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol. Hum. Reprod. 20(4):293–308, 2014.
Ksiazkiewicz, L. K. Recent achievements in in vitro culture and preservation of ovarian follicles in mammals. Reprod. Biol. 6(1):3–16, 2006.
Luyckx, V., et al. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res 6(1):83, 2013.
Mehta, J., et al. Analysis of matrix proteins, growth factors and membrane surface in commercial available freeze-dried amniotic membrane. Investig. Ophthalmol. Vis. Sci. 49(13):5745, 2008.
Merk, F. B., and N. S. McNutt. Nexus junctions between dividing and interphase granulosa cells of the rat ovary. J. Cell Biol. 55(2):511–515, 1972.
Niknejad, H., et al. Human amniotic epithelial cells induce apoptosis of cancer cells: a new anti-tumor therapeutic strategy. Cytotherapy 16(1):33–40, 2014.
Nottola, S. A., et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil. Steril. 90(1):23–32, 2008.
Nottola, S. A., et al. Ultrastructure of isolated mouse ovarian follicles cultured in vitro. Reprod. Biol. Endocrinol. 9:3, 2011.
Riau, A. K., et al. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 31(2):216–225, 2010.
Shea, L. D., T. K. Woodruff, and A. Shikanov. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 16:29–52, 2014.
Shikanov, A., et al. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J. Vis. Exp. 49:e2695, 2011.
Smitz, J., et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum. Reprod. Update 16(4):395–414, 2010.
Sun, C. C., et al. Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells 24(9):2130–2139, 2006.
Tagler, D., et al. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol. Bioeng. 111(7):1417–1429, 2014.
Van den Hurk, R., et al. Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum Reprod Update 6(5):457–474, 2000.
Wang, F., et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med. Sci. Monit. 20:2430–2438, 2014.
Wang, T. R., et al. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum. Reprod. 29(3):568–576, 2014.
West, E. R., L. D. Shea, and T. K. Woodruff. Engineering the follicle microenvironment. Semin. Reprod. Med. 25(4):287–299, 2007.
West-Farrell, E. R., et al. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol. Reprod. 80(3):432–439, 2009.
Yuan, J., et al. Transplantation of human adipose stem cell-derived hepatocyte-like cells with restricted localization to liver using acellular amniotic membrane. Stem Cell Res. Ther. 6:217, 2015.
Zhang, D., M. Jiang, and D. Miao. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS ONE 6(2):e16789, 2011.
Acknowledgment
The authors are grateful to colleagues of Royan institute histology department (Tehran, Iran) and Arash hospital (Tehran, Iran) for respectively histologic technical supports and providing human amniotic membranes. We also special thank Elham Abed Heidari for her kindly educations and advices on molecular laboratory procedures and Leila Sadat Tahaei for her guides and supports in student laboratory of Royan institute and Mohammad Jafari Atrabi for his primary laboratory educations. At the end we gratefully acknowledge Dr. Lakshmi Gopal (Valardocs Company) for the expert editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Motamed, M., Sadr, Z., Valojerdi, M.R. et al. Tissue Engineered Human Amniotic Membrane Application in Mouse Ovarian Follicular Culture. Ann Biomed Eng 45, 1664–1675 (2017). https://doi.org/10.1007/s10439-017-1836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1836-2