Abstract
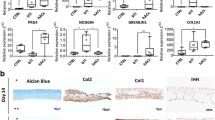
Hypertrophic chondrocytes play a critical role in endochondral bone formation as well as the progress of osteoarthritis (OA). An in vitro cartilage hypertrophy model can be used as a platform to study complex molecular mechanisms involved in these processes and screen new drugs for OA. To develop an in vitro cartilage hypertrophy model, we treated a tissue-engineered cartilage template, living hyaline cartilaginous graft (LhCG), with osteogenic medium for hypertrophic induction. In addition, endothelial progenitor cells (EPCs) were seeded onto LhCG constructs to mimic vascular invasion. The results showed that osteogenic treatment significantly inhibited the synthesis of endostatin in LhCG constructs and enhanced expression of hypertrophic marker-collagen type X (Col X) and osteogenic markers, as well as calcium deposition in vitro. Upon subcutaneous implantation, osteogenic medium-treated LhCG constructs all stained positive for Col X and showed significant calcium deposition and blood vessel invasion. Col X staining and calcium deposition were most obvious in osteogenic medium-treated only group, while there was no difference between EPC-seeded and non-seeded group. These results demonstrated that osteogenic treatment was of the primary factor to induce hypertrophic transition of LhCG constructs and this model may contribute to the establishment of an in vitro cartilage hypertrophy model.








Similar content being viewed by others
Reference
Asahara, T., T. Murohara, A. Sullivan, M. Silver, R. vanderZee, B. Witzenbichler, G. Schatteman, J. M. Isner, and M. Silver. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967, 1997.
Bahney, C. S., D. P. Hu, T. Miclau, and R. S. Marcucio. The multifaceted role of the vasculature in endochondral fracture repair. Front. Endocrinol. (Lausanne) 6:4, 2015.
Bahney, C. S., D. P. Hu, A. J. Taylor, F. Ferro, H. M. Britz, B. Hallgrimsson, B. Johnstone, T. Miclau, and R. S. Marcucio. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J. Bone Miner. Res. 29:1269–1282, 2014.
Chang, N. J., C. F. Lam, C. C. Lin, W. L. Chen, C. F. Li, Y. T. Lin, and M. L. Yeh. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthr. Cartil. 21:1613–1622, 2013.
Chen-An, P., K. V. Andreassen, K. Henriksen, M. A. Karsdal, and A. C. Bay-Jensen. Investigation of chondrocyte hypertrophy and cartilage calcification in a full-depth articular cartilage explants model. Rheumatol. Int. 33:401–411, 2013.
Filip, A., A. Bianchi, D. Mainard, P. Lacolley, J. Magdalou, and N. Mercier. A simple two dimensional culture method to study the hypertrophic differentiation of rat articular chondrocytes. Bio-Med. Mater. Eng. 25:S87–S102, 2015.
Finkenzeller, G., N. Torio-Padron, A. Momeni, A. T. Mehlhorn, and G. B. Stark. In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues. Tissue Eng. 13:1413–1420, 2007.
Freeman, F. E., M. G. Haugh, and L. M. McNamara. An in vitro bone tissue regeneration strategy combining chondrogenic and vascular priming enhances the mineralization potential of mesenchymal stem cells in vitro while also allowing for vessel formation. Tissue Eng. Part A 21:1320–1332, 2015.
Fu, W. L., Z. Xiang, F. G. Huang, Z. P. Gu, X. X. Yu, S. Q. Cen, G. Zhong, X. Duan, and M. Liu. Coculture of peripheral blood-derived mesenchymal stem cells and endothelial progenitor cells on strontium-doped calcium polyphosphate scaffolds to generate vascularized engineered bone. Tissue Eng. Part A 21:948–959, 2015.
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423:332–336, 2003.
Kusumbe A. P., S. K. Ramasamy and R. H. Adams. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone (vol 507, pg 323, 2014). Nature 513: 2014.
Lau, T. T., L. Q. P. Lee, B. N. Vo, K. Su, and D. A. Wang. Inducing ossification in an engineered 3D scaffold-free living cartilage template. Biomaterials 33:8406–8417, 2012.
Little, D. G., M. Ramachandran, and A. Schindeler. The anabolic and catabolic responses in bone repair. J. Bone Joint Surg. Br. 89:425–433, 2007.
Ma, B., J. C. H. Leijten, L. Wu, M. Kip, C. A. van Blitterswijk, J. N. Post, and M. Karperien. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr. Cartil. 21:599–603, 2013.
Murasawa, S., and T. Asahara. Endothelial progenitor cells for vasculogenesis. Physiology 20:36–42, 2005.
Nuttelman, C. R., M. C. Tripodi, and K. S. Anseth. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J. Biomed. Mater. Res. Part A 68A:773–782, 2004.
OReilly, M. S., T. Boehm, Y. Shing, N. Fukai, G. Vasios, W. S. Lane, E. Flynn, J. R. Birkhead, B. R. Olsen, and J. Folkman. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285, 1997.
Patra, D., and L. J. Sandell. Antiangiogenic and anticancer molecules in cartilage. Expert Rev. Mol. Med. 2012. doi:10.1017/erm.2012.3.
Pesesse, L., C. Sanchez, J. P. Delcour, A. Bellahcene, C. Baudouin, P. Msika, and Y. Henrotin. Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthr. Cartil. 21:1913–1923, 2013.
Pufe, T., W. J. Petersen, N. Miosge, M. B. Goldring, R. Mentlein, D. J. Varoga, and B. N. Tillmann. Endostatin/collagen XVIII—an inhibitor of angiogenesis—is expressed in cartilage and fibrocartilage. Matrix Biol. 23:267–276, 2004.
Ramasamy S. K., A. P. Kusumbe, L. Wang and R. H. Adams. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507: 376-+, 2014.
Scotti, C., E. Piccinini, H. Takizawa, A. Todorov, P. Bourgine, A. Papadimitropoulos, A. Barbero, M. G. Manz, and I. Martin. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 110:3997–4002, 2013.
Scotti, C., B. Tonnarelli, A. Papadimitropoulos, A. Scherberich, S. Schaeren, A. Schauerte, J. Lopez-Rios, R. Zeller, A. Barbero, and I. Martin. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 107:7251–7256, 2010.
Sheehy, E. J., T. Vinardell, M. E. Toner, C. T. Buckley, and D. J. Kelly. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. PLoS One 9:e90716, 2014.
Shi, Y., G. Kramer, A. Schroder, C. J. Kirkpatrick, A. Seekamp, H. Schmidt, and S. Fuchs. Early endothelial progenitor cells as a source of myeloid cells to improve the pre-vascularisation of bone constructs. Eur. Cells Mater. 27:64–80, 2014.
Sieminski, A. L., R. P. Hebbel, and K. J. Gooch. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 11:1332–1345, 2005.
Su, K., T. T. Lau, W. Y. Leong, Y. H. Gong, and D. A. Wang. Creating a living hyaline cartilage graft free from non-cartilaginous constituents: an intermediate role of a biomaterial scaffold. Adv. Funct. Mater. 22:972–978, 2012.
Sun, M. M., and F. Beier. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. C Embryo Today 102:74–82, 2014.
Terramani, T. T., D. Eton, P. A. Bui, Y. Wang, F. A. Weaver, and H. Yu. Human macrovascular endothelial cells: optimization of culture conditions. Vitro Cell. Dev. Biol.-Anim. 36:125–132, 2000.
Tsang, K. Y., D. Chan, and K. S. E. Cheah. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev. Growth Differ. 57:179–192, 2015.
Yang, G., L. Zhu, N. Hou, Y. Lan, X. M. Wu, B. Zhou, Y. Teng, and X. Yang. Osteogenic fate of hypertrophic chondrocytes. Cell Res. 24:1266–1269, 2014.
Yang, L., K. Y. Tsang, H. C. Tang, D. Chan, and K. S. E. Cheah. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 111:12097–12102, 2014.
Yoder, M. C. Human endothelial progenitor cells. Cold Spring Harbor Perspect. Med. 2:a006692, 2012.
Zhou, X., K. von der Mark, S. Henry, W. Norton, H. Adams, and B. de Crombrugghe. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10:e1004820, 2014.
Zigdon-Giladi, H., T. Bick, D. Lewinson, and E. E. Machtei. Mesenchymal stem cells and endothelial progenitor cells stimulate bone regeneration and mineral density. J. Periodontol. 85:984–990, 2014.
Zigdon-Giladi, H., T. Bick, E. F. Morgan, D. Lewinson, and E. E. Machtei. Peripheral blood-derived endothelial progenitor cells enhance vertical bone formation. Clin. Implant Dent. Relat. Res. 17:83–92, 2015.
Acknowledgments
The work was financially supported by Academic Research Fund Tier 2 (AcRF: ARC1/13), Ministry of Education, Singapore and the Natural Science Foundation of China (51328301).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael S. Detamore oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, J.Y., Lim, S.Y., He, P.F. et al. Osteogenic Treatment Initiating a Tissue-Engineered Cartilage Template Hypertrophic Transition. Ann Biomed Eng 44, 2957–2970 (2016). https://doi.org/10.1007/s10439-016-1615-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1615-5