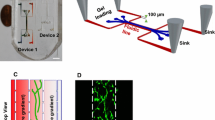
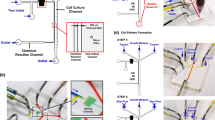
Abstract
Endothelial cells (ECs) line the interior of blood and lymphatic vessels and experience spatially varying wall shear stress (WSS) as an intrinsic part of their physiological function. How ECs, and mammalian cells generally, sense spatially varying WSS remains poorly understood, due in part to a lack of convenient tools for exposing cells to spatially varying flow patterns. We built a multiplexed device, termed a 6-well impinging flow chamber, that imparts controlled WSS gradients to a six-well tissue culture plate. Using this device, we investigated the migratory response of lymphatic microvascular ECs, umbilical vein ECs, primary fibroblasts, and epithelial cells to WSS gradients on hours to days timescales. We observed that lymphatic microvascular ECs migrate upstream, against the direction of flow, a response that was unique among all the cells types investigated here. Time-lapse, live cell imaging revealed that the microtubule organizing center relocated to the upstream side of the nucleus in response to the applied WSS gradient. To further demonstrate the utility of our device, we screened for the involvement of canonical signaling pathways in mediating this upstream migratory response. These data highlight the importance of WSS magnitude and WSS spatial gradients in dictating the cellular response to fluid flow.





Similar content being viewed by others
References
Allee, W. C. An experimental analysis of the relation between physiological states and rheotaxis in isopoda. J. Exp. Zool. 13:269–344, 1912.
Chiu, J. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91(1):327–387, 2011.
Chiu, J. J., D. L. Wang, S. Chien, R. Skalak, and S. Usami. Effects of disturbed flow on endothelial cells. J. Biomech. Eng. 120(1):2–8, 1998.
Chung, T. H., S. M. Wang, Y. C. Chang, Y. L. Chen, and J. C. Wu. 18beta-glycyrrhetinic acid promotes src interaction with connexin43 in rat cardiomyocytes. J. Cell. Biochem. 100(3):653–664, 2007.
Coan, D. E., A. R. Wechezak, R. F. Viggers, and L. R. Sauvage. Effect of shear stress upon localization of the Golgi apparatus and microtubule organizing center in isolated cultured endothelial cells. J. Cell Sci. 104(Pt 4):1145–1153, 1993.
DePaola, N., P. F. Davies, W. F. Pritchard, Jr, L. Florez, N. Harbeck, and D. C. Polacek. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc. Natl. Acad. Sci. USA 96(6):3154–3159, 1999.
Depaola, N., M. A. Gimbrone, P. F. Davies, and C. F. Dewey, Jr. Vascular endothelium responds to fluid shear-stress gradients. Arterioscler. Thromb. 12(11):1254–1257, 1992.
Depaola, N., M. A. Gimbrone, P. F. Davies, and C. F. Dewey, Jr. Vascular endothelium responds to fluid shear-stress gradients (Vol 12, Pg 1254–1257, 1992). Arterioscler. Thromb. 13(3):465–465, 1993.
Dolan, J. M., H. Meng, S. Singh, R. Paluch, and J. Kolega. High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment. Ann. Biomed. Eng. 39(6):1620–1631, 2011.
Francis, R., X. Xu, H. Park, C. J. Wei, S. Chang, B. Chatterjee, and C. Lo. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One 6(10):e26379, 2011.
Guan, X., S. Wilson, K. K. Schlender, and R. J. Ruch. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18 beta-glycyrrhetinic acid. Mol. Carcinog. 16(3):157–164, 1996.
Hove, J. R., R. W. Köster, A. S. Forouhar, G. Acevedo-Bolton, S. E. Fraser, and M. Gharib. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421(6919):172–177, 2003.
Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377(6549):539–544, 1995.
Koo, M. A., J. K. Kang, M. H. Lee, H. J. Seo, B. J. Kwon, K. E. You, M. S. Kim, D. Kim and J. C. Park. Stimulated migration and penetration of vascular endothelial cells into poly (l-lactic acid) scaffolds under flow conditions. Biomater. Res. 18(7), 2014.
LaMack, J. A., and M. H. Friedman. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am. J. Physiol.-Heart C 293(5):H2853–H2859, 2007.
Lucitti, J. L., E. A. Jones, C. Huang, J. Chen, S. E. Fraser, and M. E. Dickinson. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134(18):3317–3326, 2007.
Masuda, M., and K. Fujiwara. The biased lamellipodium development and microtubule organizing center position in vascular endothelial cells migrating under the influence of fluid flow. Biol. Cell 77(3):237–245, 1993.
Mitra, S. K., D. A. Hanson, and D. D. Schlaepfer. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6(1):56–68, 2005.
Mohamied, Y., E. M. Rowland, E. L. Bailey, S. J. Sherwin, M. A. Schwartz, and P. D. Weinberg. Change of direction in the biomechanics of atherosclerosis. Ann. Biomed. Eng. 43:16–25, 2014.
Mohan, S., N. Mohan, A. J. Valente, and E. A. Sprague. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am. J. Physiol. 276(5 Pt 1):C1100–C1107, 1999.
Muthard, R. W., and S. L. Diamond. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip 13(10):1883–1891, 2013.
Ostrowski, M. A., N. F. Huang, T. W. Walker, T. Verwijlen, C. Poplawski, A. S. Khoo, J. P. Cooke, G. G. Fuller, and A. R. Dunn. Microvascular endothelial cells migrate upstream and align against the shear stress field created by impinging flow. Biophys. J. 106(2):366–374, 2014.
Palazzo, A. F., C. H. Eng, D. D. Schlaepfer, E. E. Marcantonio, and G. G. Gundersen. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303(5659):836–839, 2004.
Paszek, M. J., N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, D. A. Hammer, and V. M. Weaver. Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254, 2005.
Polacheck, W. J., A. E. German, A. Mammoto, D. E. Ingber, and R. D. Kamm. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. USA 111(7):2447–2452, 2014.
Reinhart-King, C. A., M. Dembo, and D. A. Hammer. Cell–cell mechanical communication through compliant substrates. Biophys. J. 95(12):6044–6051, 2008.
Remuzzi, A., C. F. Dewey, Jr, P. F. Davies, and M. A. Gimbrone, Jr. Orientation of endothelial cells in shear fields in vitro. Biorheology 21(4):617–630, 1984.
Rogers, K. A., N. H. McKee, and V. I. Kalnins. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc. Natl. Acad. Sci. USA 82(10):3272–3276, 1985.
Rouleau, L., M. Farcas, J. C. Tardif, R. Mongrain, and R. L. Leask. Endothelial cell morphologic response to asymmetric stenosis hemodynamics: effects of spatial wall shear stress gradients. J. Biomech. Eng.-T Asme 8:081013, 2010.
Sakamoto, N., N. Saito, X. Han, T. Ohashi, and M. Sato. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem. Biophys. Res. Commun. 395(2):264–269, 2010.
Sato, M., N. Saito, N. Sakamoto, and T. Ohashi. High wall shear stress gradient suppress morphological responses of endothelial cells to fluid flow. IFMBE Proc. 25:312–313, 2010.
Schedin, P., and P. J. Keely. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3(1):a003228, 2011.
Schindelin, J., I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J. Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak, and A. Cardona. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9(7):676–682, 2012.
Szymanski, M. P., E. Metaxa, H. Meng, and J. Kolega. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann. Biomed. Eng. 36(10):1681–1689, 2008.
Theveneau, E., L. Marchant, S. Kuriyama, M. Gull, B. Moepps, M. Parsons, and R. Mayor. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell 19(1):39–53, 2010.
Ting, L. H., J. R. Jahn, J. I. Jung, B. R. Shuman, S. Feghhi, S. J. Han, M. L. Rodriguez, and N. J. Sniadecki. Flow mechanotransduction regulates traction forces, intercellular forces, and adherens junctions. Am. J. Physiol. Heart Circ. Physiol. 302(11):H2220–H2229, 2012.
Tomar, A., and D. D. Schlaepfer. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 21(5):676–683, 2009.
Tsou, J. K., R. M. Gower, H. J. Ting, U. Y. Schaff, M. F. Insana, A. G. Passerini, and S. I. Simon. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation 15(4):311–323, 2008.
Tzima, E., M. Irani-Tehrani, W. B. Kiosses, E. Dejana, D. A. Schultz, B. Engelhardt, G. Cao, H. DeLisser, and M. A. Schwartz. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437(7057):426–431, 2005.
Weber, G. F., M. A. Bjerke, and D. W. DeSimone. A mechanoresponsive cadherin–keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22(1):104–115, 2012.
Yoshikawa, N., H. Ariyoshi, Y. Aono, M. Sakon, T. Kawasaki, and M. Monden. Gradients in cytoplasmic calcium concentration ([Ca 2+] i) in migrating human umbilical vein endothelial cells (HUVECs) stimulated by shear-stress. Life Sci. 65(24):2643–2651, 1999.
Acknowledgments
This work was supported in part by a National Institutes of Health (NIH) New Innovator Award 1DP2OD007078-01 (A.R.D.), NIH R01HL128779 (A.R.D. and G.G.F.), and a Burroughs-Wellcome Career Award at the Scientific Interface (A.R.D.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Aleksander S. Popel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Ostrowski, M.A., Huang, E.Y., Surya, V.N. et al. Multiplexed Fluid Flow Device to Study Cellular Response to Tunable Shear Stress Gradients. Ann Biomed Eng 44, 2261–2272 (2016). https://doi.org/10.1007/s10439-015-1500-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1500-7