Abstract
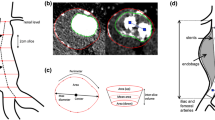
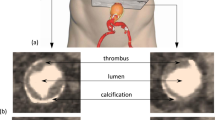
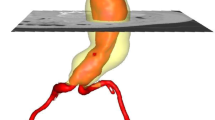
Rupture of abdominal aortic aneurysms (AAA) is responsible for 1–3% of all deaths among the elderly population in developed countries. A novel endograft proposes an endovascular aneurysm sealing (EVAS) system that isolates the aneurysm wall from blood flow using a polymer-filled endobag that surrounds two balloon-expandable stents. The volume of injected polymer is determined by monitoring the endobag pressure but the final AAA expansion remains unknown. We conceived and developed a fully deformable surface model for the comparison of pre-operative sac lumen size and final endobag size (measured using a follow-up scan) with the volume of injected polymer. Computed tomography images were acquired for eight patients. Aneurysms were manually and automatically segmented twice by the same observer. The injected polymer volume resulted 9% higher than the aneurysm pre-operative lumen size (p < 0.05), and 11% lower than the final follow-up endobag volume (p < 0.01). The automated method required minimal user interaction; it was fast and used a single set of parameters for all subjects. Intra-observer and manual vs. automated variability of measured volumes were 0.35 ± 2.11 and 0.07 ± 3.04 mL, respectively. Deformable surface models were used to quantify AAA size and showed that EVAS system devices tended to expand the sac lumen size.






Similar content being viewed by others
References
Auer, M., T. C. Gasser, K. Hållfasthetslära, and T. Skolan. Reconstruction and finite element mesh generation of abdominal aortic aneurysms from computerized tomography angiography data with minimal user interactions. IEEE Trans. Med. Imaging 31(4):1022–1028, 2010.
Ayyalasomayajula, A., A. Polk, A. Basudhar, S. Missoum, L. Nissim, and J. P. Vande Geest. Three dimensional active contours for the reconstruction of abdominal aortic aneurysms. Ann. Biomed. Eng. 38(1):164–176, 2010.
Bieniek, A., and A. Moga. An efficient watershed algorithm based on connected components. Pattern Recogn. 33(6):907–916, 2000.
Bland, J. M., and D. G. Altman. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307, 1986.
Bockler, D., A. Holden, M. Thompson, P. Hayes, D. Krievins, J. P. de Vries, and M. M. Reijnen. Multicenter Nellix endovascular aneurysm sealing system experience in aneurysm sac sealing. J. Vasc. Surg. 62(2):290–298, 2015.
Bouaziz, S., A. Tagliasacchi, and M. Pauly. Sparse iterative closest point. Comput. Graph. Forum 32(5):113–123, 2013.
Buijs, R. V., T. P. Willems, R. A. Tio, H. H. Boersma, I. F. Tielliu, R. H. Slart, and C. J. Zeebregts. Current state of experimental imaging modalities for risk assessment of abdominal aortic aneurysm. J. Vasc. Surg. 57(3):851–859, 2013.
Cignoni, P., C. Rocchini, and R. Scopigno. Metro: measuring error on simplified surfaces. Comput. Graph. Forum 17(2):167–174, 1998.
Di Martino, E. S., G. Guadagni, A. Fumero, G. Ballerini, R. Spirito, P. Biglioli, and A. Redaelli. Fluid-structure interaction within realistic three-dimensional models of the aneurysmatic aorta as a guidance to assess the risk of rupture of the aneurysm. Med. Eng. Phys. 23(9):647–655, 2001.
Donayre, C. E., C. K. Zarins, D. K. Krievins, A. Holden, A. Hill, C. Calderas, J. Velez, and R. A. White. Initial clinical experience with a sac-anchoring endoprosthesis for aortic aneurysm repair. J. Vasc. Surg. 53(3):574–582, 2011.
Duquette, A. A., P.-M. Jodoin, O. Bouchot, and A. Lalande. 3D segmentation of abdominal aorta from CT-scan and MR images. Comput. Med. Imaging Graph. 36(4):294, 2012.
Gao, L., D. G. Heath, and E. K. Fishman. Abdominal image segmentation using three-dimensional deformable models. Invest. Radiol. 33(6):348–355, 1998.
Gasser, T. C., G. Gorgulu, M. Folkesson, and J. Swedenborg. Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J. Vasc. Surg. 48(1):179–188, 2008.
Johnston, K. W., R. B. Rutherford, M. D. Tilson, D. M. Shah, L. Hollier, and J. C. Stanley. Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 13(3):452, 1991.
Kaladji, A., A. Lucas, G. Kervio, P. Haigron, and A. Cardon. Sizing for endovascular aneurysm repair: clinical evaluation of a new automated three-dimensional software. Ann. Vasc. Surg. 24(7):912–920, 2010.
Kanaoka, Y., S. Ohgi, and T. Mori. Quantitative evaluation of abdominal aortic aneurysm. Vasc. Endovasc. Surg. 33(1):59–65, 1999.
Karthikesalingam, A., R. J. Cobb, A. Khoury, E. C. Choke, R. D. Sayers, P. J. Holt, and M. M. Thompson. The morphological applicability of a novel endovascular aneurysm sealing (EVAS) system (Nellix) in patients with abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 46(4):440–445, 2013.
Kass, M., A. Witkin, and D. Terzopoulos. Snakes: active contour models. Int. J. Comput. Vision 1(4):321–331, 1988.
Kim, H. C., Y. H. Seol, S. Y. Choi, J. S. Oh, M. G. Kim, and K. Sun. A study of AAA image segmentation technique using geometric active contour model with morphological gradient edge function. Conf. Proc. IEEE Eng. Med. Biol. Soc. 4437–4440:2007, 2007.
Kniemeyer, H. W., T. Kessler, P. U. Reber, H. B. Ris, H. Hakki, and M. K. Widmer. Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? Prediction of outcome using a multi-organ-dysfunction score. Eur. J. Vasc. Endovasc. Surg. 19(2):190–196, 2000.
Krievins, D. K., A. Holden, J. Savlovskis, C. Calderas, C. E. Donayre, F. L. Moll, B. Katzen, and C. K. Zarins. EVAR using the Nellix sac-anchoring endoprosthesis: treatment of favourable and adverse anatomy. Eur. J. Vasc. Endovasc. Surg. 42(1):38–46, 2011.
Lederle, F. A., J. A. Freischlag, T. C. Kyriakides, F. T. Padberg, Jr., J. S. Matsumura, T. R. Kohler, P. H. Lin, J. M. Jean-Claude, D. F. Cikrit, K. M. Swanson, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA 302:1535–1542, 2009.
Lee, E. Region Filling Using Two Dimensional Grammars. Proc. 1987 IEEE Int. Conf. Robot Autom. pp. 147–1478, 1987.
Miller, J., D. Breen, W. Lorensen, R. O’Bara, and M. Wozny. Geometrically deformed models: a method for extracting closed geometric models form volume data. ACM 25:217–226, 1991.
Montagnat, J., H. Delingette, and N. Ayache. A review of deformable surfaces: topology, geometry and deformation. Image Vis. Comput. 19(14):1023–1040, 2001.
O’Leary, S. A., E. G. Kavanagh, P. A. Grace, T. M. McGloughlin, and B. J. Doyle. The biaxial mechanical behaviour of abdominal aortic aneurysm intraluminal thrombus: classification of morphology and the determination of layer and region specific properties. J. Biomech. 47(6):1430–1437, 2014.
Park, J.-Y., T. McInerney, D. Terzopoulos, and M.-H. Kim. A non-self-intersecting adaptive deformable surface for complex boundary extraction from volumetric images. Comput. Graph. 25(3):421–440, 2001.
Sacks, M. S., D. A. Vorp, M. L. Raghavan, M. P. Federle, and M. W. Webster. In vivo three-dimensional surface geometry of abdominal aortic aneurysms. Ann. Biomed. Eng. 27(4):469–479, 1999.
Sakalihasan, N., R. Limet, and O. D. Defawe. Abdominal aortic aneurysm. Lancet 365(9470):1577–1589, 2005.
Shum, J., G. Martufi, E. Di Martino, C. B. Washington, J. Grisafi, S. C. Muluk, and E. A. Finol. Quantitative assessment of abdominal aortic aneurysm geometry. Ann. Biomed. Eng. 39(1):277–286, 2011.
Terzopoulos, D., A. Witkin, and M. Kass. Constraints on deformable models: recovering 3D shape and nonrigid motion. Artif. Intell. 36(1):91–123, 1988.
Vande Geest, J. P., M. S. Sacks, and D. A. Vorp. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J. Biomech. 39:1324–1334, 2006.
Wever, J. J., J. D. Blankensteijn, W. P. T. M. Mali, and B. C. Eikelboom. Maximal aneurysm diameter follow-up is inadequate after endovascular abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 20(2):177–182, 2000.
Subasic M., Loncaric S, Fau-Sorantin E., and Sorantin E. 3-D image analysis of abdominal aortic aneurysm. (0926-9630 (Print)).
Acknowledgements
The authors thank Eng. Sandra Wray for her invaluable help in the writing of this manuscript. This work was partially supported by the project PIP Number 112-200901-00734 (CONICET).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Estefanía Peña oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure Supplementary Material I
(a) Dimensionless pressure as a function of time, where the dashed line shows a stable ≈0 pressure indicating the end of the GDM inflation process. (b) A detail of the pressure curve showing the intervals Tadapt and Tsizing (TIFF 105 kb)
Appendix
Appendix
GDM Initialization
The geometrically deformed model (GDM) starts with a regular icosahedron of unit side length. Each of its triangular faces goes through two processes: projection on a sphere of radius r, and subdivision in four new faces. This process is repeated until the average side length is less than a value \(d_{\text{thresh}}\), chosen in order to compromise the amount of model faces, segmentation time, and the spatial resolution of the GDM. The resulting spheroid has approximately \(\frac{\pi }{\sqrt 3 }\left( {\frac{r}{{d_{\text{thresh}} }}} \right)^{2}\) initial faces.
Vertex Normal Computation
The vertex normal vector \(\varvec{n}\) is the area-weighted sum of the unitary normal vectors to all faces that share the same vertex:
where \(\widehat{\varvec{n}}_{\varvec{j}}^{\varvec{F}}\) is the unitary normal vector of the j-th adjacent face, and \(a_{j}\) is the face area. The value of \(\widehat{\varvec{n}}_{\varvec{j}}^{\varvec{F}}\) can be computed by cross-product:
where \(\varvec{x} = \left( {x,y,z} \right)\) is the vertex position, and \(\Delta \varvec{x}_{1,j}\) and \(\Delta \varvec{x}_{2,j}\) are the vectors pointing from \(\varvec{x}\) to its two neighbor vertices in the jth adjacent face.
Inflation Force over a Vertex
The inflation force \(\varvec{f}^{\varvec{p}}\) on a vertex is approximated by the average force over all of its \(N_{F}\) adjacent faces:
Vertex Movement Simulation
Vertex movement is simulated by the kinematic equation of force-accelerated particles (Newton’s second law):
where \(m\) is the mass of the vertex, \(\varvec{f}\left( t \right)\) is the total force acting on it and \(\varvec{a}\left( t \right)\) is the resulting acceleration. The term \(\varvec{f}\left( t \right)\) at each instant is calculated as:
The term \(\varvec{f}_{\varvec{v}} \left( t \right)\) requires the prior knowledge of the vertex speed \(\varvec{v}\left( t \right)\) at that moment, which approximated from the known values in a previous instant \(t - dt\):
The future position of the vertex, \(\varvec{x}_{\varvec{f}} \left( t \right)\) is calculated for the next instant \(t + dt\):
Future position is calculated for every vertex separately, and then simultaneously updated:
GDM Parameters and Selection Criteria
Mass and damping coefficient were selected after an initial calibration experiment where the spherical GDM was inflated with a constant normal force plus a random deviation of 1% of this force. After a certain time, inflation force stopped, and the mesh was allowed to relax. The quotient \(m/\gamma = 2\) was found to stabilize the GDM in a number of iterations below T sizing with little surface oscillation (i.e., behaving like an overdamped spring system) using a unitary time step. The HU thresholds resulted from a manual seed point selection, where the mean value and standard deviation of HU were computed inside an initial sphere of 20-pixel radius. Assuming Normal and \(\chi^{2}\) distribution, the 95% confidence interval was calculated for mean HU (\(\mu\)) and standard deviation (\(\sigma\)), respectively. The upper limit was extended +6 times \(\sigma\) above the mean and the lower limit 3 times \(\sigma\) below the mean. This upper limit allowed the GDM to adequately expand through some bright structures (e.g., metallic stents or high concentrations of contrast agent). The lower limit was appropriate to constrain the GMD in the presence of dark structures surrounding the aneurysm lumen.
Rights and permissions
About this article
Cite this article
Casciaro, M.E., El-Batti, S., Chironi, G. et al. Deformable Surface Model for the Evaluation of Abdominal Aortic Aneurysms Treated with an Endovascular Sealing System. Ann Biomed Eng 44, 1381–1391 (2016). https://doi.org/10.1007/s10439-015-1446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1446-9