Abstract
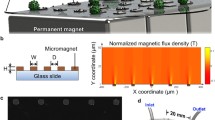
We report an inkjet-printed microscale magnetic structure that can be integrated on regular glass slides for the immunomagnetic screening of rare circulating tumor cells (CTCs). CTCs detach from the primary tumor site, circulate with the bloodstream, and initiate the cancer metastasis process. Therefore, a liquid biopsy in the form of capturing and analyzing CTCs may provide key information for cancer prognosis and diagnosis. Inkjet printing technology provides a non-contact, layer-by-layer and mask-less approach to deposit defined magnetic patterns on an arbitrary substrate. Such thin film patterns, when placed in an external magnetic field, significantly enhance the attractive force in the near-field close to the CTCs to facilitate the separation. We demonstrated the efficacy of the inkjet-print micromagnet array integrated immunomagnetic assay in separating COLO205 (human colorectal cancer cell line) from whole blood samples. The micromagnets increased the capture efficiency by 26% compared with using plain glass slide as the substrate.








Similar content being viewed by others
References
Chen, P., Y.-Y. Huang, K. Hoshino, and X. Zhang. Multiscale immunomagnetic enrichment of circulating tumor cells: from tubes to microchips. Lab Chip 14:446–458, 2014.
Chen, P., Y.-Y. Huang, K. Hoshino, and J. X. J. Zhang. Microscale magnetic field modulation for enhanced capture and distribution of rare circulating tumor cells. Sci. Rep. 5:8745, 2015.
Chen, Y., P. Li, P.-H. Huang, Y. Xie, J. D. Mai, L. Wang, N.-T. Nguyen, and T. J. Huang. Rare cell isolation and analysis in microfluidics. Lab Chip 14:626–645, 2014.
Dempsey, N. M., D. Le Roy, H. Marelli-Mathevon, G. Shaw, A. Dias, R. B. G. Kramer, L. Viet Cuong, M. Kustov, L. F. Zanini, C. Villard, K. Hasselbach, C. Tomba, and F. Dumas-Bouchiat. Micro-magnetic imprinting of high field gradient magnetic flux sources. Appl. Phys. Lett. 104:262401, 2014.
Fuller, S. B., E. J. Wilhelm, and J. M. Jacobson. Ink-jet printed nanoparticle microelectromechanical systems. J. Microelectromech. Syst. 11:54–60, 2002.
Gonzalez-Macia, L., A. Morrin, M. R. Smyth, and A. J. Killard. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analyst 135:845–867, 2010.
Hoshino, K., P. Chen, Y.-Y. Huang, and X. Zhang. Computational analysis of microfluidic immunomagnetic rare cell separation from a particulate blood flow. Anal. Chem. 84:4292–4299, 2012.
Hoshino, K., Y.-Y. Huang, N. Lane, M. Huebschman, J. W. Uhr, E. P. Frenkel, and X. Zhang. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip 11:3449–3457, 2011.
Hou, H. W., M. E. Warkiani, B. L. Khoo, Z. R. Li, R. A. Soo, D. S. W. Tan, W. T. Lim, J. Han, A. A. S. Bhagat, and C. T. Lim. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 3:1259, 2013.
Huang, Y. Y., K. Hoshino, P. Chen, C. H. Wu, N. Lane, M. Huebschman, H. Liu, K. Sokolov, J. W. Uhr, and E. P. Frenkel. Immunomagnetic nanoscreening of circulating tumor cells with a motion controlled microfluidic system. Biomed. Microdevices 15(4):673–681, 2013.
Jensen, G. C., C. E. Krause, G. A. Sotzing, and J. F. Rusling. Inkjet-printed gold nanoparticle electrochemical arrays on plastic. Application to immunodetection of a cancer biomarker protein. Phys. Chem. Chem. Phys. 13:4888–4894, 2011.
Kim, J. D., J. S. Choi, B. S. Kim, Y. C. Choi, and Y. W. Cho. Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer 51:2147–2154, 2010.
Mitchell, M. J., E. Wayne, K. Rana, C. B. Schaffer, and M. R. King. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. 111:930–935, 2014.
Nagrath, S., L. V. Sequist, S. Maheswaran, D. W. Bell, D. Irimia, L. Ulkus, M. R. Smith, E. L. Kwak, S. Digumarthy, and A. Muzikansky. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450:1235–1239, 2007.
Nawarathna, D., N. Norouzi, J. McLane, H. Sharma, N. Sharac, T. Grant, A. Chen, S. Strayer, R. Ragan, and M. Khine. Shrink-induced sorting using integrated nanoscale magnetic traps. Appl. Phys. Lett. 102:063504, 2013.
Ozkumur, E., A. M. Shah, J. C. Ciciliano, B. L. Emmink, D. T. Miyamoto, E. Brachtel, M. Yu, P.-I. Chen, B. Morgan, and J. Trautwein. Inertial focusing for tumor antigen–dependent and–independent sorting of rare circulating tumor cells. Sci. Trans. Med. 5:179ra47, 2013.
Riethdorf, S., H. Fritsche, V. Müller, T. Rau, C. Schindlbeck, B. Rack, W. Janni, C. Coith, K. Beck, and F. Jänicke. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the Cell Search system. Clin. Cancer Res. 13:920–928, 2007.
Seekamp, J., S. Zankovych, A. Helfer, P. Maury, C. S. Torres, G. Boettger, C. Liguda, M. Eich, B. Heidari, and L. Montelius. Nanoimprinted passive optical devices. Nanotechnology 13:581, 2002.
Shields, I. V., C. Wyatt, C. D. Reyes, and G. P. López. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 15(5):1230–1249, 2015.
Singh, M., H. M. Haverinen, P. Dhagat, and G. E. Jabbour. Inkjet printing—process and its applications. Adv. Mater. 22:673–685, 2010.
Singh, M., H. M. Haverinen, P. Dhagat, and G. E. Jabbour. Inkjet printing-process and its applications. Adv. Mater. 22:673, 2010.
Stott, S. L., C.-H. Hsu, D. I. Tsukrov, M. Yu, D. T. Miyamoto, B. A. Waltman, S. M. Rothenberg, A. M. Shah, M. E. Smas, and G. K. Korir. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. 107:18392–18397, 2010.
Tekin, E., P. J. Smith, S. Hoeppener, A. M. van den Berg, A. S. Susha, A. L. Rogach, J. Feldmann, and U. S. Schubert. Inkjet printing of luminescent CdTe nanocrystal–polymer composites. Adv. Funct. Mater. 17:23–28, 2007.
Tekin, E., P. J. Smith, and U. S. Schubert. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 4:703–713, 2008.
Truskett, V. N., and M. P. Watts. Trends in imprint lithography for biological applications. Trends Biotechnol. 24:312–317, 2006.
Voit, W., W. Zapka, L. Belova, and K. Rao. Application of inkjet technology for the deposition of magnetic nanoparticles to form micron-scale structures. IEE Proc. Sci. Meas. Technol. 150:252–256, 2003.
Williams, S. C. P. Circulating tumor cells. Proc. Natl. Acad. Sci. 110:4861, 2013.
Wu, W., G.-Y. Jung, D. Olynick, J. Straznicky, Z. Li, X. Li, D. Ohlberg, Y. Chen, S.-Y. Wang, and J. Liddle. One-kilobit cross-bar molecular memory circuits at 30-nm half-pitch fabricated by nanoimprint lithography. Appl. Phys. A 80:1173–1178, 2005.
Yung, C. W., J. Fiering, A. J. Mueller, and D. E. Ingber. Micromagnetic-microfluidic blood cleansing device. Lab Chip 9:1171–1177, 2009.
Zanini, L. F., N. M. Dempsey, D. Givord, G. Reyne, and F. Dumas-Bouchiat. Autonomous micro-magnet based systems for highly efficient magnetic separation. Appl. Phys. Lett. 99:232504, 2011.
Acknowledgment
We thank our collaborator Professor Konstantin V. Sokolov at the University of Texas MD Anderson Cancer Center and Dr. Zhigang Li at the Geisel School of Medicine at Dartmouth for the supports in validating the presented results. We thank Ms. Nancy Lane and Drs. Michael Huebschman, Jonathan W. Uhr, and Eugene P. Frenkel at the University of Texas Southwestern Medical Center for their invaluable suggestions on the experiment design. We appreciate the help from Professor Tim Yeh and Dr. Judy Obliosca at the University of Texas at Austin with the spectrofluorometer measurement. We are grateful for the financial support from the National Institute of Health (NIH) National Cancer Institute (NCI) Cancer Diagnosis Program under Grant 1R01CA139070.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Chen, P., Huang, YY., Bhave, G. et al. Inkjet-Print Micromagnet Array on Glass Slides for Immunomagnetic Enrichment of Circulating Tumor Cells. Ann Biomed Eng 44, 1710–1720 (2016). https://doi.org/10.1007/s10439-015-1427-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1427-z