Abstract
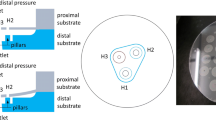
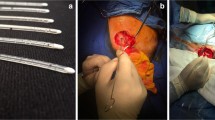
Reliable cerebrospinal fluid (CSF) draining methods are needed to treat hydrocephalus, a chronic debilitating brain disorder. Current shunt implant treatments are characterized by high failure rates that are to some extent attributed to their length and multiple components. The designed valve, made of hydrogel, steers away from such protracted schemes and intends to provide a direct substitute for faulty arachnoid granulations, the brain’s natural CSF draining valves, and restore CSF draining operations within the cranium. The valve relies on innate hydrogel swelling phenomena to strengthen reverse flow sealing at idle and negative pressures thereby alleviating common valve failure mechanisms. In vitro measurements display operation in range of natural CSF draining (cracking pressure, P T ~ 1–110 mmH2O and outflow hydraulic resistance, R h ~ 24–152 mmH2O/mL/min), with negligible reverse flow leakage (flow, Q O > −10 µL/min). Hydrodynamic measurements and over-time tests under physically relevant conditions further demonstrate the valve’s operationally-reproducible properties and strengthen its validity for use as a chronic implant.







Similar content being viewed by others
References
Brodbelt, A., and M. Stoodley. CSF pathways: a review. Brit. J. Neurosurg. 21(5):510–520, 2007.
Bruus, H. Theoretical Microfluidics. New York: Oxford University Press, 2007.
Brydon, H. L., R. Bayston, R. Hayward, and W. Harkness. The effect of protein and blood cells on the flow-pressure characteristics of shunts. Neurosurgery 38(3):498–505, 1996.
Brydon, H. L., G. Keir, E. J. Thompson, R. Bayston, R. Hayward, and W. Harkness. Protein adsorption to hydrocephalus shunt catheters: CSF protein adsorption. J. Neurol. Neurosurg. PS. 64(5):643–647, 1998.
Chabrerie, A., and P. M. Black. Ventricular shunts. J. Intensive Care Med. 17(5):218–229, 2002.
Codman, “Hydrocephalus Catalog,” 2006. [Online]. Available: http://implantesclp.com/uploads/pdf/Codman.pdf. [Accessed 4 October 2014].
Czosnyka, Z. H., K. Cieslicki, M. Czosnyka, and J. D. Pickard. Hydrocephalus shunts and waves of intracranial pressure. Med. Bio. Eng. Comput. 43(1):71–77, 2005.
Czosnyka, M., Z. Czosnyka, H. Whitehouse, and J. D. Pickard. Hydrodynamic properties of hydrocephalus shunts: United Kingdom shunt evaluation laboratory. J. Neurol. Neurosurg. Psychiatry 62(1):43–50, 1997.
Drake, J. M. M. B., J. R. M. Kestle, and R. M. Milner. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 43(2):294–303, 1998.
Drake, J. M., J. R. W. Kestle, and S. Tuli. CSF shunts 50 years on—past, present, and future. Childs Nerv. Syst. 16:10–11, 2000.
Elixmann, I. M., M. Kwiecien, C. Goffin, M. Walter, B. Misgeld, M. Keifer, W.-I. Steudel, K. Radermacher, and S. Leonhardt. Control of an electromechanical hydrocephalus shunt—a new approach. IEEE. Trans. Biomed. Eng. 61(9):2379–2388, 2014.
Garrett, Q., B. Laycock, and R. W. Garrett. Hydrogel lens monomer constituents modulate protein sorption. Invest. Ophth. Vis. Sci. 41(7):1687–1695, 2000.
Gehrke, S. H., G. P. Andrews, and E. L. Cussler. Chemical aspects of gel extraction. Chem. Eng. Sci. 41(8):2153–2160, 1986.
Hassler, C., T. Boretius, and T. Stieglitz. Polymers for neural implants. J. Polym. Sci. Part B 49(1):18–33, 2010.
Je, S. S., and J. Chae. A compact, low-power, and electromagnetically actuated microspeaker for hearing aids. IEEE Electron Device Lett. 29:856–858, 2008.
Johansson, S. B., A. Eklund, J. Malm, G. Stemme, and N. Roxhed. A MEMS-based passive hydrocephalus shunt for body position controlled intracranial pressure regulation. Biomed. Microdevices 16:529–536, 2014.
Kim, D., and D. Beebe. A bi-polymer micro one-way valve. Sens. Actuators A 136:426–433, 2007.
Kotzar, G., M. Freas, P. Abel, A. Fleischman, S. Roy, C. Zorman, J. M. Moran, and J. Melzak. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 23(13):2737–2750, 2002.
Lesho, M. J., and N. F. Sheppard, Jr. Adhesion of polymer films to oxidized silicon and its effect on performance of a conductometric pH sensor. Sensors Actuators B 37:61–66, 1996.
Li, H., T. Y. Ng, Y. K. Yew, and K. Y. Lam. Modeling and simulation of the swelling behavior of pH-stimulus-responsive hydrogels. Biomacromolecules 6(1):109–120, 2004.
Lo, R., P. Li, S. Saati, R. N. Agrawal, M. S. Humayan, and E. Meng. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 11:959–970, 2009.
Medtronic, “Neurological Products: Shunts,” Medtronic, 8 Oct 2012. [Online]. Available: http://www.medtronic.com/for-healthcare-professionals/products-therapies/neurological/shunts/index.htm. [Accessed 25 May 2014].
Montheard, J., M. Chatzopoulos, and D. Chappard. 2-Hydroxyethyl methacrylate (HEMA): chemical properties and applications in biomedical fields. J. Macromol. Sci. Part C 32(1):1–34, 1992.
Oh, J., G. Kim, F. Kralick, and H. Noh. Design and fabrication of a PDMS/parylene microvalve for the treatment of hydrocephalus. IEEE/ASME J. Microelectromech. Syst. 20(4):811–818, 2011.
Quddos, A., H. U. Rehaman, A. Wadood, S. Sulfiqar, and M. L. Mirza. The effect of crosslinking agents on the synthesis and swelling of the polymer networks. J. Chem. Soc. Pak. 25(4):299–304, 2003.
Reddy, G. K., P. Bollam, and G. Caldito. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 81(2):404–410, 2013.
Reiber, H. Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122:189–203, 1994.
Schwerdt, H. N., R. Bristol, and J. Chae. Miniaturized passive hydrogel check valve for hydrocephalus treatment. IEEE Trans. Biomed. Eng. 61(3):814–820, 2014.
Stone, J. J., C. T. Walker, M. Jacobson, V. Phillips, and H. J. Silberstein. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J. Neurosurg Pediatrics 11(1):15–19, 2013.
Timoschenko, S. Theory of Plates and Shells. New York: McGraw-Hill, 1940.
Tirumala, V. R., R. Divan, D. C. Mancini, and G. T. Caneba. Fabrication of high-aspect-ratio hydrogel microstructures. Microsyst. Technol. 11:347–352, 2005.
Tranoudis, I., and N. Efron. Tensile properties of soft contact lens materials. Contact Lens Anterior Eye 27(4):177–191, 2004.
Yang, Y., and J. Chae. Miniaturized protein separation using a liquid chromatography column on a flexible substrate. J. Micromech. Microeng. 18:125010, 2008.
Yetkin, F., U. Kayabas, Y. Ersoy, Y. Bayinder, S. A. Toplu, and I. Tek. Cerebrospinal fluid viscosity: a novel diagnostic measure for acute meningitis. Southern Med. J. 103(9):892–895, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Andreas Anayiotos oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Schwerdt, H.N., Amjad, U., Appel, J. et al. In Vitro Hydrodynamic, Transient, and Overtime Performance of a Miniaturized Valve for Hydrocephalus. Ann Biomed Eng 43, 603–615 (2015). https://doi.org/10.1007/s10439-015-1291-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1291-x