Abstract
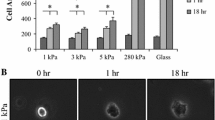
Infiltrating leukocytes are exposed to a wide range of tissue elasticities. While we know the effects of substrate elasticity on acute inflammation via the study of neutrophil migration, we do not know its effects on leukocytes that direct chronic inflammatory events. Here, we studied morphology and motility of macrophages, the innate immune cells that orchestrate acute and chronic inflammation, on polyacrylamide hydrogels that mimicked a wide range of tissue elasticities. As expected, we found that macrophage spreading area increased as substrate elasticity increased. Unexpectedly, we found that morphology did not inversely correlate with motility. In fact, velocity of steady-state macrophages remained unaffected by substrate elasticity, while velocity of biologically stimulated macrophages was limited on stiff substrates. We also found that the lack of motility on stiff substrates was due to a lack of lipid rafts on the leading edge of the macrophages. This study implicates lipid rafts in the mechanosensory mechanism of innate immune cell infiltration.





Similar content being viewed by others
References
Baldewsing, R. A., J. A. Schaar, F. Mastik, and A. F. van der Steen. Local elasticity imaging of vulnerable atherosclerotic coronary plaques. Adv. Cardiol. 44:35–61, 2007.
Beningo, K. A., M. Dembo, I. Kaverina, J. V. Small, and Y. L. Wang. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153:881–888, 2001.
Byfield, F. J., H. Aranda-Espinoza, V. G. Romanenko, G. H. Rothblat, and I. Levitan. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys. J. 87:3336–3343, 2004.
de Korte, C. L., A. F. van der Steen, E. I. Cepedes, G. Pasterkamp, S. G. Carlier, F. Mastik, A. H. Schoneveld, P. W. Serruys, and N. Bom. Characterization of plaque components and vulnerability with intravascular ultrasound elastography. Phys. Med. Biol. 45:1465–1475, 2000.
Elkin, B. S., A. I. Ilankovan, and B. Morrison, 3rd. A detailed viscoelastic characterization of the p17 and adult rat brain. J. Neurotrauma 28:2235–2244, 2011.
Engler, A. J., C. Carag-Krieger, C. P. Johnson, M. Raab, H. Y. Tang, D. W. Speicher, J. W. Sanger, J. M. Sanger, and D. E. Discher. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 121:3794–3802, 2008.
Fraser, I. P., H. Koziel, and R. A. Ezekowitz. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10:363–372, 1998.
Georges, P. C., W. J. Miller, D. F. Meaney, E. S. Sawyer, and P. A. Janmey. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 90:3012–3018, 2006.
Gomez-Mouton, C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and A. C. Martinez. Segregation of leading-edge and uropod components into specific lipid rafts during t cell polarization. Proc. Natl. Acad. Sci. USA 98:9642–9647, 2001.
Gordon, S. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927–930, 2002.
Isenberg, B. C., P. A. Dimilla, M. Walker, S. Kim, and J. Y. Wong. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 97:1313–1322, 2009.
Jannat, R. A., M. Dembo, and D. A. Hammer. Traction forces of neutrophils migrating on compliant substrates. Biophys. J. 101:575–584, 2011.
Jannat, R. A., G. P. Robbins, B. G. Ricart, M. Dembo, and D. A. Hammer. Neutrophil adhesion and chemotaxis depend on substrate mechanics. J. Phys. 22:194117, 2010.
Jeon, J. H., S. K. Kim, H. J. Kim, J. Chang, C. M. Ahn, and Y. S. Chang. Lipid raft modulation inhibits nsclc cell migration through delocalization of the focal adhesion complex. Lung Cancer 69:165–171, 2012.
Kleveta, G., K. Borzecka, M. Zdioruk, M. Czerkies, H. Kuberczyk, N. Sybirna, A. Sobota, and K. Kwiatkowska. Lps induces phosphorylation of actin-regulatory proteins leading to actin reassembly and macrophage motility. J. Cell. Biochem. 113:80–92, 2012.
Lauridsen, H. M., B. J. Walker, and A. L. Gonzalez. Chemically- and mechanically-tunable porated polyethylene glycol gels for leukocyte integrin independent and dependent chemotaxis. Technology 2:133–143, 2014.
Leitinger, B., and N. Hogg. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115:963–972, 2002.
Levental, I., P. Georges, and P. Janmey. Soft biological materials and their impact on cell function. Soft Tissue Matter 3:299–306, 2007.
Lo, C. M., H. B. Wang, M. Dembo, and Y. L. Wang. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79:144–152, 2000.
Manes, S., E. Mira, C. Gomez-Mouton, R. A. Lacalle, P. Keller, J. P. Labrador, and A. C. Martinez. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18:6211–6220, 1999.
Matsumoto, T., H. Abe, T. Ohashi, Y. Kato, and M. Sato. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol. Meas. 23:635–648, 2002.
Oakes, P. W., D. C. Patel, N. A. Morin, D. P. Zitterbart, B. Fabry, J. S. Reichner, and J. X. Tang. Neutrophil morphology and migration are affected by substrate elasticity. Blood 114:1387–1395, 2009.
Previtera, M. Mechanotransduction in the immune system. Cell. Mol. Bioeng. 2014. doi:10.1007/s12195-014-0338-7.
Previtera, M. L., M. Hui, D. Verma, A. J. Shahin, R. Schloss, and N. A. Langrana. The effects of substrate elastic modulus on neural precursor cell behavior. Ann. Biomed. Eng. 41:1193–1207, 2013.
Previtera, M. L., C. G. Langhammer, and B. L. Firestein. Effects of substrate stiffness and cell density on primary hippocampal cultures. J. Biosci. Bioeng. 110:459–470, 2010.
Previtera, M. L., C. G. Langhammer, N. A. Langrana, and B. L. Firestein. Regulation of dendrite arborization by substrate stiffness is mediated by glutamate receptors. Ann. Biomed. Eng. 38:3733–3743, 2010.
Schaar, J. A., C. L. De Korte, F. Mastik, C. Strijder, G. Pasterkamp, E. Boersma, P. W. Serruys, and A. F. Van Der Steen. Characterizing vulnerable plaque features with intravascular elastography. Circulation 108:2636–2641, 2003.
Solon, J., I. Levental, K. Sengupta, P. C. Georges, and P. A. Janmey. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93:4453–4461, 2007.
Stroka, K. M., and H. Aranda-Espinoza. Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil. Cytoskelet. 66:328–341, 2009.
Tracqui, P., A. Broisat, J. Toczek, N. Mesnier, J. Ohayon, and L. Riou. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J. Struct. Biol. 174:115–123, 2011.
Triantafilou, M., K. Miyake, D. T. Golenbock, and K. Triantafilou. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603–2611, 2002.
Vassilieva, E. V., K. Gerner-Smidt, A. I. Ivanov, and A. Nusrat. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am. J. Physiol. 295:G965–G976, 2008.
Wang, R., J. Bi, K. K. Ampah, X. Ba, W. Liu, and X. Zeng. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. Biochim. Biophys. Acta 3195–3205:2013, 1833.
Wang, R., J. Bi, K. K. Ampah, C. Zhang, Z. Li, Y. Jiao, X. Wang, X. Ba, and X. Zeng. Lipid raft regulates the initial spreading of melanoma a375 cells by modulating beta1 integrin clustering. Int. J. Biochem. Cell Biol. 45:1679–1689, 2013.
Wickstrom, S. A., K. Alitalo, and J. Keski-Oja. Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of RhoA activity. J. Biol. Chem. 278:37895–37901, 2003.
Wuerfel, J., F. Paul, B. Beierbach, U. Hamhaber, D. Klatt, S. Papazoglou, F. Zipp, P. Martus, J. Braun, and I. Sack. Mr-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage 49:2520–2525, 2010.
Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 60:24–34, 2005.
Zhang, X., R. Goncalves, and D. M. Mosser. The isolation and characterization of murine macrophages (Chap. 14: Unit 14). Curr. Protoc. Immunol. 11:14, 2008.
Acknowledgments
Thank you to Mason Hui and Norell Hadzimichalis for editing this manuscript. Funding was provided by the JFK Neuroscience Institute and the JFK Foundation.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Previtera, M.L., Peterman, K., Shah, S. et al. Lipid Rafts Direct Macrophage Motility in the Tissue Microenvironment. Ann Biomed Eng 43, 896–905 (2015). https://doi.org/10.1007/s10439-014-1142-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1142-1