Abstract
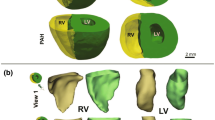
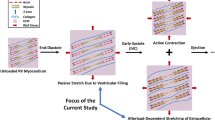
Right ventricular (RV) failure in response to pulmonary hypertension (PH) is a severe disease that remains poorly understood. PH-induced pressure overload leads to changes in the RV free wall (RVFW) that eventually results in RV failure. While the development of computational models can benefit our understanding of the onset and progression of PH-induced pressure overload, detailed knowledge of the underlying structural and biomechanical events remains limited. The goal of the present study was to elucidate the structural and biomechanical adaptations of RV myocardium subjected to sustained pressure overload in a rat model. Hemodynamically confirmed severe chronic RV pressure overload was induced in Sprague–Dawley rats via pulmonary artery banding. Extensive tissue-level biaxial mechanical and histomorphological analyses were conducted to assess the remodeling response in the RV free wall. Simultaneous myofiber hypertrophy and longitudinal re-orientation of myo- and collagen fibers were observed, with both fiber types becoming more highly aligned. Transmural myo- and collagen fiber orientations were co-aligned in both the normal and diseased state. The overall tissue stiffness increased, with larger increases in longitudinal vs. circumferential stiffness. The latter was attributed to longitudinal fiber re-orientation, which increased the degree of anisotropy. Increased mechanical coupling between the two axes was attributed to the increased fiber alignment. Interestingly, estimated myofiber stiffness increased while the collagen fiber stiffness remained unchanged. The increased myofiber stiffness was consistent with clinical results showing titin-associated increased sarcomeric stiffening observed in PH patients. These results further our understanding of the underlying adaptive and maladaptive remodeling mechanisms and may lead to improved techniques for prognosis, diagnosis, and treatment for PH.








Similar content being viewed by others
Abbreviations
- RV:
-
Right ventricle
- RVFW:
-
RV free wall
- PA:
-
Pulmonary artery
- RVESP:
-
RV end-systolic pressure
- RVEDV:
-
RV end-diastolic volume
- SV:
-
Stroke volume
- SW:
-
Stroke work
- Maximum dP/dt :
-
Maximal first time-derivative of pressure (a measure of systolic function)
- Tau:
-
RV diastolic time constant (a measure of diastolic function)
- Ees :
-
RV elastance (a measure of contractility)
- Ea :
-
PA elastance (a measure of afterload)
- Ees/Ea :
-
RV–PA coupling
- E LL :
-
Green’s strain in longitudinal direction
- E CC :
-
Green’s strain in circumferential direction
- S LL :
-
2nd Piola–Kirchhoff (PK) stress in longitudinal direction
- S CC :
-
2nd PK stress in circumferential direction
- b 0 :
-
Model scaling parameter
- b L :
-
Model parameter, representing longitudinal stiffness
- b C :
-
Model parameter, representing circumferential stiffness
- b LC :
-
Model parameter, representing coupling, between longitudinal and circumferential response
- S ens :
-
Combined myofiber-collagen effective fiber ensemble stress
- E ens :
-
Combined myofiber-collagen effective fiber ensemble strain
- \(\Phi_{\text{m}}\) :
-
Mass fraction of myofibers
- \(\Phi_{\text{c}}\) :
-
Mass fraction of collagen fibers
- \(\eta_{\text{m}}\) :
-
Intrinsic myofiber modulus
- \(\bar{\eta }_{\text{c}}\) :
-
Intrinsic collagen fiber modulus, that accounts for the effects of gradual fiber recruitment
- E lb :
-
Lower bound on recruitment strain for the collagen fiber ensemble
- E ub :
-
Upper bound on recruitment strain for the collagen fiber ensemble
- PTTM:
-
Post-transition tangent modulus
References
Archer, S. L., and E. D. Michelakis. An evidence-based approach to the management of pulmonary arterial hypertension. Curr. Opin. Cardiol. 21(4):385–392, 2006.
Benza, R. L., M. H. Park, A. Keogh, and R. E. Girgis. Management of pulmonary arterial hypertension with a focus on combination therapies. J. Heart Lung Transplant. 26(5):437–446, 2007.
Billiar, K. L., and M. S. Sacks. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II–A structural constitutive model. J. Biomech. Eng. 122(4):327–335, 2000.
Bogaard, H. J., K. Abe, A. Vonk Noordegraaf, and N. F. Voelkel. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135(3):794–804, 2009.
Brand, M. D., and D. G. Nicholls. Assessing mitochondrial dysfunction in cells. Biochem. J. 435(2):297–312, 2011.
Bristow, M. R., L. S. Zisman, B. D. Lowes, W. T. Abraham, D. B. Badesch, B. M. Groves, N. F. Voelkel, D. M. Lynch, and R. A. Quaife. The pressure-overloaded right ventricle in pulmonary hypertension. Chest 114(1 Suppl):101S–106S, 1998.
Campo, A., S. C. Mathai, J. Le Pavec, A. L. Zaiman, L. K. Hummers, D. Boyce, T. Housten, H. C. Champion, N. Lechtzin, F. M. Wigley, R. E. Girgis, and P. M. Hassoun. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 182(2):252–260, 2010.
Carlsson, M., M. Ugander, E. Heiberg, and H. Arheden. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am. J. Physiol. Heart Circ. Physiol. 293(1):H636–H644, 2007.
Caulfield, J. B., and T. K. Borg. The collagen network of the heart. Lab. Investig. 40(3):364–372, 1979.
Champion, H. C., E. D. Michelakis, and P. M. Hassoun. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 120(11):992–1007, 2009.
Chance, B., and G. R. Williams. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 217(1):383–393, 1955.
Choi, H. S., and R. P. Vito. Two-dimensional stress–strain relationship for canine pericardium. J. Biomech. Eng. 112(2):153–159, 1990.
Chuong, C. J., M. S. Sacks, G. Templeton, F. Schwiep, and R. L. Johnson, Jr. Regional deformation and contractile function in canine right ventricular free wall. Am. J. Physiol. 260(4 Pt 2):H1224–H1235, 1991.
Cohn, J. N., R. Ferrari, and N. Sharpe. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 35(3):569–582, 2000.
Courtney, T., M. S. Sacks, J. Stankus, J. Guan, and W. R. Wagner. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 27(19):3631–3638, 2006.
D’Alonzo, G. E., R. J. Barst, S. M. Ayres, E. H. Bergofsky, B. H. Brundage, K. M. Detre, A. P. Fishman, R. M. Goldring, B. M. Groves, J. T. Kernis, P. S. Levy, G. G. Pietra, L. M. Reid, J. T. Reeves, S. Rich, C. E. Vreim, G. W. Williams, and M. Wu. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 115(5):343–349, 1991.
Eriksson, T. S., A. J. Prassl, G. Plank, and G. A. Holzapfel. Modeling the dispersion in electromechanically coupled myocardium. Int. J. Numer. Method Biomed. Eng. 29(11):1267–1284, 2013.
Faber, M. J., M. Dalinghaus, I. M. Lankhuizen, P. Steendijk, W. C. Hop, R. G. Schoemaker, D. J. Duncker, J. M. Lamers, and W. A. Helbing. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure–volume loops. Am. J. Physiol. Heart Circ. Physiol. 291(4):H1580–H1586, 2006.
Fata, B., W. Zhang, R. Amini, and M. Sacks. Insights into regional adaptations in the growing pulmonary artery using a meso-scale structural model: effects of ascending aorta impingement. J. Biomech. Eng. 136(2):021009, 2014.
Fisher, M. R., P. R. Forfia, E. Chamera, T. Housten-Harris, H. C. Champion, R. E. Girgis, M. C. Corretti, and P. M. Hassoun. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 179(7):615–621, 2009.
Forfia, P. R., M. R. Fisher, S. C. Mathai, T. Housten-Harris, A. R. Hemnes, B. A. Borlaug, E. Chamera, M. C. Corretti, H. C. Champion, T. P. Abraham, R. E. Girgis, and P. M. Hassoun. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 174(9):1034–1041, 2006.
Forfia, P. R., S. C. Mathai, M. R. Fisher, T. Housten-Harris, A. R. Hemnes, H. C. Champion, R. E. Girgis, and P. M. Hassoun. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 177(12):1364–1369, 2008.
Freed, A. D., D. R. Einstein, and I. Vesely. Invariant formulation for dispersed transverse isotropy in aortic heart valves: an efficient means for modeling fiber splay. Biomech. Model. Mechanobiol. 4(2–3):100–117, 2005.
Fung, Y. C. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer, 1993.
Galie, N., M. M. Hoeper, M. Humbert, A. Torbicki, J. L. Vachiery, J. A. Barbera, M. Beghetti, P. Corris, S. Gaine, J. S. Gibbs, M. A. Gomez-Sanchez, G. Jondeau, W. Klepetko, C. Opitz, A. Peacock, L. Rubin, M. Zellweger, and G. Simonneau. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 30(20):2493–2537, 2009.
Gasser, T. C., R. W. Ogden, and G. A. Holzapfel. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3(6):15–35, 2006.
George, M. P., H. C. Champion, and J. M. Pilewski. Lung transplantation for pulmonary hypertension. Pulm. Circ. 1(2):182–191, 2011.
Grossman, W., D. Jones, and L. P. McLaurin. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 56(1):56–64, 1975.
Grossman, W., and W. J. Paulus. Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling. J. Clin. Investig. 123(9):3701–3703, 2013.
Hemnes, A. R., and H. C. Champion. Right heart function and haemodynamics in pulmonary hypertension. Int. J. Clin. Pract. 62:11–19, 2008.
Holzapfel, G. A. Nonlinear Solid Mechanics: A Continuum Approach for Engineering. New York: Wiley, 2000.
Holzapfel, G. A., and T. C. Gasser. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61:1–48, 2000.
Horowitz, A., Y. Lanir, F. C. Yin, M. Perl, I. Sheinman, and R. K. Strumpf. Structural three-dimensional constitutive law for the passive myocardium. J. Biomech. Eng. 110(3):200–207, 1988.
Humphrey, J. D., and K. R. Rajagopal. A constrained mixture model for growth and remodeling of soft tissues. Math. Models Methods Appl. Sci. 12(3):407–430, 2002.
Jammalamadaka, S. R., and A. Sengupta. Topics in Circular Statistics. River Edge, NJ: World Scientific, 2001.
Janicki, J. S., G. L. Brower, J. D. Gardner, M. F. Forman, J. A. Stewart, Jr., D. B. Murray, and A. L. Chancey. Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc. Res. 69(3):657–665, 2006.
Karlon, W. J., J. W. Covell, A. D. McCulloch, J. J. Hunter, and J. H. Omens. Automated measurement of myofiber disarray in transgenic mice with ventricular expression of ras. Anat. Rec. 252(4):612–625, 1998.
Kass, D. J., E. Rattigan, R. Kahloon, K. Loh, L. Yu, A. Savir, M. Markowski, A. Saqi, R. Rajkumar, F. Ahmad, and H. C. Champion. Early treatment with fumagillin, an inhibitor of methionine aminopeptidase-2, prevents Pulmonary Hypertension in monocrotaline-injured rats. PLoS ONE 7(4):e35388, 2012.
Kuehne, T., S. Yilmaz, P. Steendijk, P. Moore, M. Groenink, M. Saaed, O. Weber, C. B. Higgins, P. Ewert, E. Fleck, E. Nagel, I. Schulze-Neick, and P. Lange. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation 110(14):2010–2016, 2004.
Lin, D. H., and F. C. Yin. A multiaxial constitutive law for mammalian left ventricular myocardium in steady-state barium contracture or tetanus. J. Biomech. Eng. 120(4):504–517, 1998.
Mahan, R. P. Circular Statistical Methods: Applications in Spatial and Temporal Performance Analysis. Special Report 16. Alexandria, Virginia, United States Army Research Institute for the Behavioral and Social Sciences, 1991.
Maughan, W. L., A. A. Shoukas, K. Sagawa, and M. L. Weisfeldt. Instantaneous pressure–volume relationship of the canine right ventricle. Circ. Res. 44(3):309–315, 1979.
Onat, E. T., and F. A. Leckie. Representation of mechanical-behavior in the presence of changing internal structure. J. Appl. Mech. Trans. ASME 55(1):1–10, 1988.
Rain, S., M. L. Handoko, P. Trip, C. T. Gan, N. Westerhof, G. J. Stienen, W. J. Paulus, C. A. Ottenheijm, J. T. Marcus, P. Dorfmuller, C. Guignabert, M. Humbert, P. Macdonald, C. Dos Remedios, P. E. Postmus, C. Saripalli, C. G. Hidalgo, H. L. Granzier, A. Vonk-Noordegraaf, J. van der Velden, and F. S. de Man. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128(18):2016–2025; 1-10, 2013.
Sacks, M. Biaxial mechanical evaluation of planar biological materials. J. Elast. 61:199–246, 2000.
Sacks, M. S. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125(2):280–287, 2003.
Sacks, M. S., and C. J. Chuong. Orthotropic mechanical properties of chemically treated bovine pericardium. Ann. Biomed. Eng. 26(5):892–902, 1998.
Sanz, J., A. Garcia-Alvarez, L. Fernandez-Friera, A. Nair, J. G. Mirelis, S. T. Sawit, S. Pinney, and V. Fuster. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 98(3):238–243, 2012.
Streeter, Jr., D. D., H. M. Spotnitz, D. P. Patel, J. Ross, Jr., and E. H. Sonnenblick. Fiber orientation in the canine left ventricle during diastole and systole. Circ. Res. 24(3):339–347, 1969.
Suga, H., and K. Sagawa. Instantaneous pressure–volume relationships and their ratio in the excised, supported canine left ventricle. Circ. Res. 35(1):117–126, 1974.
Sugden, P. H., and A. Clerk. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. (Berl.) 76(11):725–746, 1998.
Sun, W., M. S. Sacks, T. L. Sellaro, W. S. Slaughter, and M. J. Scott. Biaxial mechanical response of bioprosthetic heart valve biomaterials to high in-plane shear. J. Biomech. Eng. 125:372–380, 2003.
Tedford, R. J., J. O. Mudd, R. E. Girgis, S. C. Mathai, A. L. Zaiman, T. Housten-Harris, D. Boyce, B. W. Kelemen, A. C. Bacher, A. A. Shah, L. K. Hummers, F. M. Wigley, S. D. Russell, R. Saggar, R. Saggar, W. L. Maughan, P. M. Hassoun, and D. A. Kass. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ. Heart Fail. 6(5):953–963, 2013.
Tonelli, A. R., V. Arelli, O. A. Minai, J. Newman, N. Bair, G. A. Heresi, and R. A. Dweik. Causes and circumstances of death in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 188(3):365–369, 2013.
Trip, P., E. J. Nossent, F. S. de Man, I. A. van den Berk, A. Boonstra, H. Groepenhoff, E. M. Leter, N. Westerhof, K. Grunberg, H. J. Bogaard, and A. Vonk Noordegraaf. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension—patient characteristics and treatment responses. Eur. Respir. J. 42(6):1575–1585, 2013.
Valdez-Jasso, D., M. A. Simon, H. C. Champion, and M. S. Sacks. A murine experimental model for the mechanical behaviour of viable right-ventricular myocardium. J. Physiol. 590(18):4571–4584, 2012.
Voelkel, N. F., R. Natarajan, J. I. Drake, and H. J. Bogaard. Right ventricle in pulmonary hypertension. Compr. Physiol. 1(1):525–540, 2011.
Acknowledgments
This work was supported by the U.S. National Institutes of Health [1F32 HL117535 to M.R.H., P01 HL103455 and U01 HL108642-01 to H.C.C.]; the American Heart Association [13POST14720047 to M.R.H., 11POST6950004 to D.V-J., 10BGIA3790022 to M.A.S.]; and The Pittsburgh Foundation [M2010-0052 to M.A.S. and M.S.S.]. We’d like to thank Sunaina Rustagi, Andrea Sebastiani, and Samantha Carter at the University of Pittsburgh (Pitt) for performing the biomechanical testing; Jeffrey J. Baust at Pitt for performing pulmonary artery banding procedures; Sruti Shiva at Pitt for performing the tissue viability study; Simone Siegel, Michelle Atkins, and John Lesicko at the University of Texas at Austin (UT-Austin) for performing the histomorphological analysis.
Conflict of Interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Hill, M.R., Simon, M.A., Valdez-Jasso, D. et al. Structural and Mechanical Adaptations of Right Ventricle Free Wall Myocardium to Pressure Overload. Ann Biomed Eng 42, 2451–2465 (2014). https://doi.org/10.1007/s10439-014-1096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1096-3