Abstract
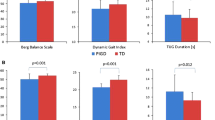
The purpose of this study was to investigate the effects of dopaminergic therapy on neuromuscular complexity during gait and on the relationship between neuromuscular complexity and gait speed in persons with Parkinson’s disease (PD). Nine persons with PD walked at self-selected speed for 5 min after having withdrawn from dopaminergic medication for at least 12 h and while optimally-medicated. Electromyographic recordings were taken from eight leg muscles bilaterally. Non-negative matrix factorization was applied to reduce the dimensionality of the electromyographic signals into motor modules. We assessed neuromuscular complexity by investigating the number, structure, and timing of the modules. We also investigated the influence of dopaminergic medication on the relationships between neuromuscular complexity and gait speed. Though gait speed increased significantly after medication intake, medication did not affect neuromuscular complexity. Neuromuscular complexity was significantly associated with gait speed only while the participants were medicated. Thus, the supraspinal structures that govern neuromuscular complexity during gait do not appear to be solely dopaminergically-influenced in PD. The lack of dopaminergic influence on neuromuscular complexity may explain why persons with PD exhibit gait slowness even while medicated, and an intervention that restores neuromuscular complexity may result in gait speed improvement in PD.





Similar content being viewed by others
References
Allen, J. L., S. A. Kautz, and R. R. Neptune. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin. Biomech. (Bristol, Avon) 28:697–704, 2013.
Allen, J. L., and R. R. Neptune. Three-dimensional modular control of human walking. J. Biomech. 45:2157–2163, 2012.
Amano, S., R. T. Roemmich, J. W. Skinner, and C. J. Hass. Ambulation and Parkinson disease. Phys. Med. Rehabil. Clin. N. Am. 24:371–392, 2013.
Birkmayer, W., and O. Hornykiewicz. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien KlinWochenschr. 73:787–788, 1961.
Blin, O., A. M. Ferrandez, J. Pailhous, and G. Serratrice. Dopa-sensitive and dopa-resistant gait parameters in Parkinson’s disease. J. Neurol. Sci. 103:51–54, 1991.
Bohnen, N. I., K. A. Frey, S. Studenski, V. Kotagal, R. A. Koeppe, P. J. Scott, R. L. Albin, and M. L. Müller. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 81:1611–1616, 2013.
Bohnen, N. I., M. L. Müller, R. A. Koeppe, S. A. Studenski, M. A. Kilbourn, K. A. Frey, and R. L. Albin. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 73:1670–1676, 2009.
Cheung, V. C., A. d’Avella, M. C. Tresch, and E. Bizzi. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J. Neurosci. 25:6419–6434, 2005.
Chvatal, S. A., J. M. Macpherson, G. Torres-Oviedo, and L. H. Ting. Absence of postural muscle synergies for balance after spinal cord transection. J. Neurophysiol. 110:1301–1310, 2005.
Clark, D. J., L. H. Ting, F. E. Zajac, R. R. Neptune, and S. A. Kautz. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 103:844–857, 2010.
Cotzias, G. C., M. H. Van Woert, and L. M. Schiffer. Aromatic amino acids and modification of parkinsonism. N. Engl. J. Med. 276:374–379, 1967.
Dingwell, J. B., and L. C. Marin. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J. Biomech. 39:444–452, 2006.
Ellis, T., J. T. Cavanaugh, G. M. Earhart, M. P. Ford, K. B. Foreman, and L. E. Dibble. Which measures of physical function and motor impairment best predict quality of life in Parkinson’s disease? Parkinsonism Relat. Disord. 17:693–697, 2011.
Giszter, S. F., M. R. Davies, and V. Graziani. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J. Neurophysiol. 97:2663–2675, 2007.
Grillner, S., P. Wallén, K. Saitoh, A. Kozlov, and B. Robertson. Neural bases of goal-directed locomotion in vertebrates–an overview. Brain Res. Rev. 57:2–12, 2008.
Hass, C. J., M. D. Bishop, M. Moskovich, E. L. Stegemöller, J. W. Skinner, I. A. Malaty, A. Wagle Shukla, K. N. McFarland, and M. S. Okun. Defining the clinically meaningful difference in gait speed in Parkinson’s disease. J. Neurol. Phys. Ther. (2014, accepted).
Hirsch, E. C., A. M. Graybiel, C. Duyckaerts, and F. Javoy-Agid. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclearpalsy. Proc. Natl Acad. Sci. U.S.A. 84:5976–5980, 1987.
Ivanenko, Y. P., G. Cappellini, N. Dominici, R. E. Poppele, and F. Lacquaniti. Coordination of locomotion with voluntary movements in humans. J. Neurosci. 25:7238–7253, 2005.
Ivanenko, Y. P., R. E. Poppele, and F. Lacquaniti. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 556:267–282, 2004.
Karachi, C., D. Grabli, F. A. Bernard, D. Tandé, N. Wattiez, H. Belaid, E. Bardinet, A. Prigent, H. P. Nothacker, S. Hunot, A. Hartmann, S. Lehéricy, E. C. Hirsch, and C. François. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 120:2745–2754, 2010.
Knutsson, E. An analysis of Parkinsonian gait. Brain 95:475–486, 1972.
Krystkowiak, P., J. L. Blatt, J. L. Bourriez, A. Duhamel, M. Perina, S. Blond, J. D. Guieu, A. Destée, and L. Defebvre. Effects of subthalamic nucleus stimulation and levodopa treatment on gait abnormalities in Parkinson disease. Arch. Neurol. 60:80–84, 2003.
Morris, M. E., R. Iansek, T. A. Matyas, and J. J. Summers. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 117:1169–1181, 1994.
Müller, M. L., R. L. Albin, V. Kotagal, R. A. Koeppe, P. J. Scott, K. A. Frey, and N. I. Bohnen. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 136:3282–3289, 2013.
Neptune, R. R., D. J. Clark, and S. A. Kautz. Modular control of human walking: a simulation study. J. Biomech. 42:1282–1287, 2009.
Pierantozzi, M., M. G. Palmieri, S. Galati, P. Stanzione, A. Peppe, D. Tropepi, L. Brusa, A. Pisani, V. Moschella, M. G. Marciani, P. Mazzone, and A. Stefani. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson’s disease patients. J. Neural Transm. 115:731–735, 2008.
Rochester, L., A. J. Yarnall, M. R. Baker, R. V. David, S. Lord, B. Galna, and D. J. Burn. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 135:2779–2788, 2012.
Rodriguez, K. L., R. T. Roemmich, B. Cam, B. J. Fregly, and C. J. Hass. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 124:1390–1397, 2013.
Roh, J., V. C. Cheung, and E. Bizzi. Modules in the brain stem and spinal cord underlying motor behaviors. J. Neurophysiol. 106:1363–1378, 2011.
Rolland, A. S., D. Tandé, M. T. Herrero, M. R. Luquin, M. Vazquez-Claverie, C. Karachi, E. C. Hirsch, and C. François. Evidence for a dopaminergic innervation of the pedunculopontine nucleus in monkeys, and its drastic reduction after MPTP intoxication. J. Neurochem. 110:1321–1329, 2009.
Routson, R. L., D. J. Clark, M. G. Bowden, S. A. Kautz, and R. R. Neptune. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 38:511–517, 2013.
Ryczko, D., S. Grätsch, F. Auclair, C. Dubé, S. Bergeron, M. H. Alpert, J. J. Cone, M. F. Roitman, S. Alford, and R. Dubuc. Forebrain dopamine neurons project down to a brainstem region controlling locomotion. Proc. Natl Acad. Sci. U.S.A. 110:3235–3242, 2013.
Shulman, L. M., A. L. Gruber-Baldini, K. E. Anderson, P. S. Fishman, S. G. Reich, and W. J. Weiner. The clinically important difference on the unified Parkinson’s disease rating scale. Arch. Neurol. 67:64–70, 2010.
Spaulding, S. J., B. Barber, M. Colby, B. Cormack, T. Mick, and M. E. Jenkins. Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis. Arch. Phys. Med. Rehabil. 94(3):562–570, 2013.
Ting, L. H., and S. A. Chvatal. Decomposing muscle activity in motor tasks: methods and interpretation. In: Motor Control: Theories, Experiments, and Applications, edited by F. Danion, and M. L. Latash. Oxford: Oxford University Press, 2010, pp. 102–138.
Ting, L. H., and J. M. Macpherson. A limited set of muscle synergies for force control during a postural task. J. Neurophysiol. 93:609–613, 2005.
Torres-Oviedo, G., J. M. Macpherson, and L. H. Ting. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 96:1530–1546, 2006.
Tresch, M. C., V. C. Cheung, and A. d’Avella. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J. Neurophysiol. 95:2199–2212, 2006.
Acknowledgments
This work was supported in part by NIH grants 1R21AG033284-01A2 and UF National Parkinson’s Foundation Center of Excellence. We would also like to thank Dr. Umer Akbar and Dr. Nawaz Hack for their assistance in scoring the UPDRS videos.
Conflict of interest
The authors declare that there are no relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Thurmon E. Lockhart oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Roemmich, R.T., Fregly, B.J. & Hass, C.J. Neuromuscular Complexity During Gait is not Responsive to Medication in Persons with Parkinson’s Disease. Ann Biomed Eng 42, 1901–1912 (2014). https://doi.org/10.1007/s10439-014-1036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1036-2