Abstract
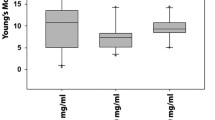
Human mesenchymal stem cells (hMSCs) are multipotent cells appropriate for a variety of tissue engineering and cell therapy applications. Mechanical properties of hMSCs during differentiation are associated with their particular metabolic activity and regulate cell function due to alternations in cytoskeleton and structural elements. The objective of this study is to evaluate elastic and viscoelastic properties of hMSCs during long term cultivation in control and transforming growth factor-β1 treatment groups using micropipette aspiration technique. The mean Young’s modulus (E) of the control samples remained nearly unchanged during 6 days of cultivation, but that of the test samples showed an initial reduction compared to its relevant control sample after 2 days of treatment by biological growth factor, followed by a significant rise after 4 and 6 days. The viscoelastic creep tests showed that both instantaneous and equilibrium moduli significantly increased with the treatment time and reached to maximum values of 622.9 ± 114.2 and 144.3 ± 11.6 Pa at the sixth day, respectively, while increase in apparent viscosity was not statistically significant. Such change of mechanical properties of hMSCs during specific lineage commitment contributes to regenerative medicine as well as stem-cell-based therapy in which biophysical signals regulate stem cell fate.




Similar content being viewed by others
References
Bianco, P., M. Riminucci, S. Granthos, and P. G. Robey. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192, 2001.
Chou, R., M. Stromer, R. Robson, and T. Huiatt. Assembly of contractile and cytoskeletal elements in developing smooth muscle cells. Dev. Biol. 149:339–348, 1992.
Derynck, R., and Y. Zhang. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature 425:577–584, 2003.
Gonzalez-Cruz, R. D., V. C. Fonseca, and E. M. Darling. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. PNAS 109:1523–1529, 2012.
Guilak, F., J. Tedrow, and R. Burgkart. Viscoelastic properties of cell nucleus. Biochem. Biophys. Res. Commun. 269:781–786, 2000.
Guilak, F., L. G. Alexopoulos, M. A. Haider, H. P. Ting-Beall, and L. A. Setton. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons Isolated by Cartilage Homogenization. Ann. Biomed. Eng. 33:1312–1318, 2005.
Guo, X., and S. Chen. Transforming growth factor-β and smooth muscle differentiation. World J. Biol. Chem. 3:41–52, 2012.
Hochmuth, R. M. Micropipette aspiration of living cells. J. Biomech. 33:15–22, 2000.
Jones, W. R., H. P. Ting-Beall, G. M. Lee, S. S. Kelley, R. M. Hochmuth, and F. Guilak. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32:119–127, 1999.
Kurpinski, K., H. Lam, J. Chu, A. Wang, A. Kim, E. Tsay, S. Agrawal, D. V. Schaffer, and S. Li. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28:734–742, 2010.
Kurpinski, K., J. Chu, D. Wang, and S. Li. Proteomic profiling of mesenchymal stem cells responses to mechanical strain and TGF-β1. Cell. Mol. Bioeng. 2:606–614, 2009.
Li, D., J. Zhou, F. Chowdhury, J. Cheng, N. Wang, and F. Wang. Role of mechanical factors in fate decisions of stem cells. Regen. Med. 6:229–240, 2011.
Lim, C. T., E. H. Zhou, and S. T. Quek. Mechanical models for living cells: a review. J. Biomech. 39:195–216, 2006.
McBeath, R., D. Pirone, C. Nelson, K. Bhadiraju, and C. S. Chen. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6:483–495, 2004.
Narita, Y., A. Yamawaki, H. Kagami, M. Ueda, and Y. Udea. Effects of transforming growth factor-beta1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 333:449–459, 2008.
Owens, G. K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75:487–517, 1995.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshakl. Multilineage potential of adult human mesenchymal stem cells. Science 284:147–151, 1999.
Rodriguez, J. P., M. Gonzalez, S. Rios, and V. Cambiazo. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 93:721–731, 2004.
Settleman, J. Tension precedes commitment-even for a stem cell. Mol. Cell 14:148–150, 2004.
Shi, Z. D., G. Abraham, and J. M. Tarbell. Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparin sulfate proteoglycans and ERK1/2. PLoS ONE 5:12196, 2010.
Stegemann, J. P., and R. M. Nerem. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 31:391–402, 2003.
Sun, H., M. Yamamoto, M. Mejillano, and H. Yin. Gelsolin, a Multifunctional Actin Regulatory Protein. J. Biol. Chem. 274:33179–33182, 1999.
Tan, S. C., W. X. Pan, G. Ma, N. Cai, K. W. Leong, and K. Liao. Viscoelastic behavior of human mesenchymal stem cells. BMC Cell Biol. 9:40, 2008.
Theret, D. P., M. J. Levesque, M. Sato, R. M. Nerem, and L. T. Wheeler. The application of a homogeneous half-space model in the analysis of endothelial-cell micropipette measurements. J. Biomech. Eng. 110:190–199, 1988.
Titushkin, I., and M. Cho. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys. J. 93:3693–3702, 2007.
Trickey, W. R., T. P. Vail, and F. Guilak. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J. Orthop. Res. 22:131–139, 2004.
Volokh, K. Y. Cytoskeletal architecture and mechanical behavior of living cells. Biorheology 40:213–220, 2003.
Wang, D., J. Park, J. Chu, A. Krakowski, K. Luo, D. Chen, and S. Li. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. J. Biol. Chem. 279:43725–43734, 2004.
Wang, J. H., and B. P. Thampatty. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 5:1–16, 2006.
Yourek, G., M. Hussain, and J. Mao. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 53:219–228, 2007.
Yu, H., C. Y. Tay, W. S. Leong, S. C. Tan, K. Liao, and L. P. Tan. Mechanical behavior of human mesenchymal stem cells during adipogenic and osteogenic differentiation. Biochem. Biophys. Res. Commun. 393:150–155, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Khani, MM., Tafazzoli-Shadpour, M., Rostami, M. et al. Evaluation of Mechanical Properties of Human Mesenchymal Stem Cells During Differentiation to Smooth Muscle Cells. Ann Biomed Eng 42, 1373–1380 (2014). https://doi.org/10.1007/s10439-013-0889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0889-0