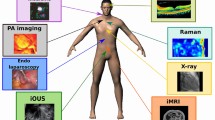
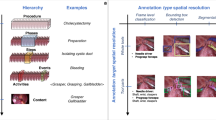
Abstract
The emergence of Minimal Access Surgery (MAS) as a paradigm in modern healthcare treatment has created new challenges and opportunities for automated image understanding and computer vision. In MAS, images recovered from inside the body using specialized devices are used to visualize and operate on the surgical site but they can also be used to computationally infer in vivo 3D tissue surface shape, soft-tissue morphology, and surgical instrument motion. This information is important for facilitating in vivo biophotonic imaging modalities where the interaction between light and tissue is used to infer the structural and functional properties of the tissue. This article provides a review of the literature for computer vision and image understanding techniques applied to MAS images. The focus of this article is to elucidate a perspective on how computer vision techniques can be used to support and enhance the capabilities of biophotonic imaging modalities during surgery. Note that while MAS encompasses a variety of surgical specializations this review does not involve procedures performed in the interventional suite. The review has been carried out based on searches in the PubMed and IEEE databases using the article’s keywords.





Similar content being viewed by others
References
Albitar, I. C., P. Graebling, and C. Doignon. Robust Structured Light Coding for 3D Reconstruction. In: IEEE 11th International Conference on Computer Vision, 2007—ICCV 2007, pp. 1–6, 2007.
Allain, B., M. Hu, L. Lovat, R. Cook, T. Vercauteren, S. Ourselin, and D. Hawkes. A System for Biopsy Site Re-targeting with Uncertainty in Gastroenterology and Oropharyngeal Examinations. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 514–521, 2010.
André, B., T. Vercauteren, A. M. Buchner, M. B. Wallace, and N. Ayache. A smart atlas for endomicroscopy using automated video retrieval. Med. Image Anal. 4:460–476, 2011.
Arnold, M., A. Ghosh, S. Ameling, and G. Lacey. Automatic segmentation and inpainting of specular highlights for endoscopic imaging. J. Image Video Process. 1–12, 2010.
Atasoy, S., D. Noonan, S. Benhimane, N. Navab, and G.-Z. Yang. A Global Approach for Automatic Fibroscopic Video Mosaicing in Minimally Invasive Diagnosis. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2008, pp. 850–857, 2008.
Atif, M., H. Ullah, M. Y. Hamza, and M. Ikram. Catheters for optical coherence tomography. Laser Phys. Lett. 9:629–646, 2011.
Bachta, W., P. Renaud, L. Cuvillon, E. Laroche, A. Forgione, and J. Gangloff. Motion prediction for computer-assisted beating heart surgery. IEEE Trans. Biomed. Eng. 11:2551–2563, 2009.
Balicki, M., J.-H. Han, I. Iordachita, P. Gehlbach, J. Handa, R. Taylor, and J. Kang. Single Fiber Optical Coherence Tomography Microsurgical Instruments for Computer and Robot-Assisted Retinal Surgery. In: Proceedings of the 12th International Conference on Medical Image Computing and Computer-Assisted Intervention: Part I, pp. 108–115, 2009.
Baumhauer, M., M. Feuerstein, H. P. Meinzer, and J. Rassweiler. Navigation in endoscopic soft tissue surgery: perspectives and limitations. J. Endourol. 4:751–761, 2008.
Becker, V., T. Vercauteren, C. H. von Weyhern, C. Prinz, R. M. Schmid, and A. Meining. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video). Gastrointest. Endosc. 5:1001–1007, 2007.
Boppart, S. A., B. E. Bouma, C. Pitris, G. J. Tearney, J. G. Fujimoto, and M. E. Brezinski. Forward-imaging instruments for optical coherence tomography. Opt. Lett. 21:1618–1620, 1997.
Bouget, J.-Y. Camera Calibration Toolkit for Matlab. http://www.vision.caltech.edu/bouguetj/calib_doc/. 2011.
Bouma, B. E., and G. J. Tearney. Power-efficient nonreciprocal interferometer and linear-scanning fiber-optic catheter for optical coherence tomography. Opt. Lett. 8:531–533, 1999.
Burschka, D., M. Li, R. Taylor, and G. D. Hager. Scale-Invariant Registration of Monocular Endoscopic Images to CT-Scans for Sinus Surgery. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 413–421, 2004.
Camarillo, D. B., T. M. Krummel, and J. K. Salisbury, Jr. Robotic technology in surgery: past, present, and future. Am. J. Surg. 4(Suppl 1):2–15, 2004.
Can, A., C. V. Stewart, B. Roysam, and H. L. Tanenbaum. A feature-based technique for joint, linear estimation of high-order image-to-mosaic transformations: application to mosaicing the curved human retina. In: IEEE Conference on Computer Vision and Pattern Recognition, Vol. 2, pp. 585–591, 2000.
Cano, A., F. Gayá, P. Lamata, P. Sánchez-González, and E. Gómez. Laparoscopic tool tracking method for augmented reality surgical applications biomedical simulation. Lect. Notes Comput. Sci. 5104:191–196, 2008.
Cech, J., J. Sanchez-Riera, and R. Horaud. Scene flow estimation by growing correspondence seeds. In: IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 2011, pp. 3129–3136, 2011.
Clancy, N. T., D. Stoyanov, V. Sauvage, D. James, G.-Z. Yang, and D. S. Elson. A Triple Endoscope System for Alignment of Multispectral Images of Moving Tissue. In: BTuD27, 2010.
Clancy, N. T., D. Stoyanov, G.-Z. Yang, and D. S. Elson. An endoscopic structured lighting probe using spectral encoding. In: Proceedings of SPIE, 2011.
Collins, T., B. Compte, and A. Bartoli. Deformable Shape-from-Motion in Laparoscopy using a Rigid Sliding Window. In: Medical Image Understanding and Analysis Conference, 2011.
Darzi, A., and S. Mackay. Recent advances in minimal access surgery. Br. Med. J. 7328:31–34, 2002.
Deguchi, K., and T. Okatani. Shape Reconstruction from an Endoscope Image by Shape- from-Shading Technique for a Point Light Source at the Projection Center. In: IEEE Workshop on Mathematical Methods in Biomedical Image Analysis, pp. 290–298, 1996.
Deligianni, F., A. Chung, and G.-z. Yang. Patient-specific bronchoscope simulation with pq-space-based 2D/3D registration. Comput. Aided Surg. 5:215–226, 2004.
Devernay, F., F. Mourgues, and E. Coste-Maniere. Towards endoscopic augmented reality for robotically assisted minimally invasive cardiac surgery. In: Medical Imaging and Augmented Reality, 2001.
Doignon, C., F. Nageotte, and M. de Mathelin. The Role of Insertion Points in the Detection and Positioning of Instruments in Laparoscopy for Robotic Tasks. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006, pp. 527–534, 2006.
dos Santos, T., A. Seitel, H.-P. Meinzer, and L. Maier-Hein. Correspondences Search for Surface-Based Intra-Operative Registration. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 660–667, 2010.
Fleming, I. N., S. Voros, B. Vagvolgyi, Z. Pezzementi, J. Handa, R. Taylor, and G. D. Hager. Intraoperative Visualization of Anatomical Targets in Retinal Surgery. In: Proceedings of the 2008 IEEE Workshop on Applications of Computer Vision, pp. 1–6, 2008.
Fuchs, H., M. A. Livingston, R. Raskar, D. Colucci, K. Keller, A. State, J. R. Crawford, P. Rademacher, S. H. Drake, and A. A. Meyer. Augmented reality visualization for laparoscopic surgery. In: Medical Image Computing and Computer-Assisted Intervention, pp. 934–943, 1998.
Ginhoux, R., J. Gangloff, M. de Mathelin, L. Soler, M. M. A. Sanchez, J. Marescaux, et al. Active filtering of physiological motion in robotized surgery using predictive control. IEEE Trans. Robot. 1:67–79, 2005.
Gioux, S., A. Mazhar, D. J. Cuccia, A. J. Durkin, B. J. Tromberg, and J. V. Frangioni. Three-dimensional surface profile intensity correction for spatially modulated imaging. J. Biomed. Opt. 14:34–45, 2009.
Grasa, Ó. G., J. Civera, and J. M. M. Montiel. EKF Monocular SLAM with Relocalization for Laparoscopic Sequences. In: IEEE International Conference on Robotics and Automation, 2011.
Gröger, M., T. Ortmaier, W. Sepp, and G. Hirzinger. Reconstruction of Image Structure in Presence of Specular Reflections. In: DAGM-Symposium on Pattern Recognition, pp. 53–60, 2001.
Guo-Qing, W., K. Arbter, and G. Hirzinger. Real-time visual servoing for laparoscopic surgery. Controlling robot motion with color image segmentation. IEEE Eng. Med. Biol. Mag. 1:40–45, 1997.
Guthart, G. S., and J. K. Salisbury. The Intuitive Telesurgery System: Overview and Application. In: IEEE International Conference on Robotics and Automation, pp. 618–621, 2000.
Hager, G., A. Okamura, P. Kazanzides, L. Whitcomb, G. Fichtinger, and R. Taylor. Surgical and interventional robotics: part III Tutorial. IEEE Robot. Autom. Mag. 4:84–93, 2008.
Hager, G., B. Vagvolgyi, and D. Yuh. Stereoscopic Video Overlay with Deformable Registration. In: Medicine Meets Virtual Reality, 2007.
Hamamoto, Y., T. Endo, K. Nosho, Y. Arimura, M. Sato, and K. Imai. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J. Gastroenterol. 1:14–20, 2004.
Hammer, D. X. Advances in Retinal Imaging. In: Advances in Optical Imaging for Clinical Medicine, pp. 85–161, 2011.
Haogang, Z., D. P. Crabb, P. G. Schlottmann, G. Wollstein, and D. F. Garway-Heath. Aligning scan acquisition circles in optical coherence tomography images of the retinal nerve fibre layer. IEEE Trans. Med. Imaging 6:1228–1238, 2011.
Hartley, R., and A. Zisserman. Multiple View Geometry in Computer Vision. Cambridge: Cambridge Press, 2000.
Heikkila, J., and O. Silven. A Four-step Camera Calibration Procedure with Implicit Image Correction. In: IEEE Computer Society Conference on Computer Vision and Pattern Recognition, pp. 1106–1112, 1997.
Helferty, J. P., and W. E. Higgins. Combined Endoscopic Video Tracking and Virtual 3D CT Registration for Surgical Guidance. In: Proceedings of the 2002 International Conference on Image Processing, Vol. 2, pp. II-961–II-964, 2002.
Hernández-Mier, Y., W. C. P. M. Blondel, C. Daul, D. Wolf, and F. Guillemin. Fast construction of panoramic images for cystoscopic exploration. Comput Med Imaging Graph 7:579–592, 2010.
Höller, K., J. Penne, A. Schneider, J. Jahn, J. Guttiérrez Boronat, T. Wittenberg, H. Feußner, and J. Hornegger. Endoscopic Orientation Correction. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2009, pp. 459–466, 2009.
Hu, M., G. Penney, P. Edwards, M. Figl, and D. Hawkes. 3D reconstruction of internal organ surfaces for minimal invasive surgery. Med. Image Comput. Comput. Assist. Interv. Int. Conf. Med. Image Comput. Comput. Assist. Interv. Pt 1:68–77, 2007.
Hu, M., G. Penney, D. Rueckert, P. Edwards, F. Bello, R. Casula, M. Figl, and D. Hawkes. Non-rigid Reconstruction of the Beating Heart Surface for Minimally Invasive Cardiac Surgery. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2009, pp. 34–42, 2009.
Huang, D., et al. Optical coherence tomography. Science 5035:1178, 1991.
Iftimia, N., W. R. Brugge, and D. X. Hammer. Advances in Optical Imaging for Clinical Medicine. Hoboken, NJ: Wiley, 2011.
Keller, K., and J. D. Ackerman. Real-time structured light depth extraction. In: Proceedings of SPIE, p. 11, 2000.
Krupa, A., J. Gangloff, C. Doignon, M. F. de Mathelin, M. Guillaume Morel, J. Leroy, L. Soler, J. Marescaux, et al. Autonomous 3-D positioning of surgical instruments in robotized laparoscopic surgery using visual servoing. IEEE Trans. Robot. Autom. 5:842–853, 2003.
Lamata, P., T. Morvan, M. Reimers, E. Samset and J. Declerck. Addressing Shading-Based Laparoscopic Registration. In: World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany, pp. 189–192, 2009.
Lau, W. W., N. A. Ramey, J. Corso, N. V. Thakor, and G. D. Hager. Stereo-Based Endoscopic Tracking of Cardiac Surface Deformation. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 494–501, 2004.
Le Goualher, G., A. Perchant, M. Genet, C. Cavé, B. Viellerobe, F. Berier, B. Abrat, and N. Ayache. Towards Optical Biopsies with an Integrated Fibered Confocal Fluorescence Microscope. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2004, pp. 761–768, 2004.
Lerotic, M., A. J. Chung, J. Clark, S. Valibeik, and G.-Z. Yang. Dynamic View Expansion for Enhanced Navigation in Natural Orifice Transluminal Endoscopic Surgery. In: Proceedings of the 11th International Conference on Medical Image Computing and Computer-Assisted Intervention, Part II, pp. 467–475, 2008.
Lerotic, M., and G. Yang. Super resolution in robotic-assisted minimally invasive surgery. Comput. Aided Surg. 6:347–356, 2007.
Loewke, K. E., D. B. Camarillo, W. Piyawattanametha, M. J. Mandella, C. H. Contag, S. Thrun, and J. K. Salisbury. In vivo micro-image mosaicing. IEEE Trans. Biomed. Eng. 1:159–171, 2011.
Loewke, K., D. Camarillo, K. Salisbury, and S. Thrun. Deformable Image Mosaicing for Optical Biopsy. In: IEEE 11th International Conference on Computer Vision, 2007 (ICCV 2007), pp. 1–8, 2007.
Lucas, B., and T. Kanade. An Iterative Image Registration Technique with an Application to Stereo Vision. In: International Joint Conference on Artificial Intelligence, pp. 674–679, 1981.
Maier-Hein, L., M. Schmidt, A. Franz, T. dos Santos, A. Seitel, B. Jähne, J. Fitzpatrick, and H. Meinzer. Accounting for Anisotropic Noise in Fine Registration of Time-of-Flight Range Data with High-Resolution Surface Data. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 251–258, 2010.
Malti, A., A. Bartoli, and T. Collins. Template-Based Conformal Shape-from-Motion from Registered Laparoscopic Images. In: Medical Image Understanding and Analysis Conference. 2011.
Mikolajczyk, K., and C. Schmid. Scale & affine invariant interest point detectors. Int. J. Comput. Vis. 1:63–86, 2004.
Mirota, D. J., M. Ishii, and G. D. Hager. Vision-based navigation in image-guided interventions. Annu. Rev. Biomed. Eng. 1:297–319, 2011.
Mirota, D., H. Wang, R. Taylor, M. Ishii, and G. Hager. Toward Video-Based Navigation for Endoscopic Endonasal Skull Base Surgery. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2009, pp. 91–99, 2009.
Mitchell, B., J. Koo, M. Iordachita, P. Kazanzides, A. Kapoor, J. Handa, G. Hager, and R. Taylor. Development and Application of a New Steady-Hand Manipulator for Retinal Surgery. In: IEEE International Conference on Robotics and Automation, 2007, pp. 623–629, 2007.
Mori, K., D. Deguchi, J. Sugiyama, Y. Suenagaa, J. Toriwakia, C. R. Maurer, Jr., H. Takabatake, and H. Natori. Tracking of a bronchoscope using epipolar geometry analysis and intensity-based image registration of real and virtual endoscopic images. Med. Image Anal. 3:321–336, 2002.
Mountney, P., S. Giannarou, D. Elson, and G.-Z. Yang. Optical Biopsy Mapping for Minimally Invasive Cancer Screening. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2009, pp. 483–490, 2009.
Mountney, P., and Y. Guang-Zhong. Dynamic view expansion for minimally invasive surgery using simultaneous localization and mapping. In: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009 (EMBC 2009), pp. 1184–1187, 2009.
Mountney, P., B. P. L. Lo, S. Thiemjarus, D. Stoyanov, and G.-Z. Yang. A Probabilistic Framework for Tracking Deformable Soft Tissue in Minimally Invasive Surgery. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 34–41, 2007.
Mountney, P., D. Stoyanov, A. Davison, and G.-Z. Yang. Simultaneous Stereoscope Localization and Soft-Tissue Mapping for Minimal Invasive Surgery. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006, pp. 347–354, 2006.
Mountney, P., D. Stoyanov, and Y. Guang-Zhong. Three-dimensional tissue deformation recovery and tracking. IEEE Signal Process. Mag. 4:14–24, 2010.
Mountney, P., and G.-Z. Yang. Motion Compensated SLAM for Image Guided Surgery. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 496–504, 2010.
Mourgues, F., F. Devernay, G. Malandain, and È. Coste-Manière. 3D reconstruction of the operating field for image overlay in 3D-endoscopic surgery. In: International Symposium on Augmented Reality. 2001.
Namati, E., J. Thiesse, J. de Ryk, and G. McLennan. In vivo assessment of alveolar morphology using a flexible catheter-based confocal microscope. IET Comput. Vis. 4:228–235, 2008.
Nasseri, S. S., J. L. Kasperbauer, S. E. Strome, T. V. McCaffrey, J. L. Atkinson, and F. B. Meyer. Endoscopic transnasal pituitary surgery: report on 180 cases. Am. J. Rhinol. 4:281–287, 2001.
Nguyen, F. T., A. M. Zysk, E. J. Chaney, S. G. Adie, J. G. Kotynek, U. J. Oliphant, F. J. Bellafiore, K. M. Rowland, P. A. Johnson, and S. A. Boppart. Optical coherence tomography: the intraoperative assessment of lymph nodes in breast cancer. IEEE Eng. Med. Biol. Mag. 2:63–70, 2010.
Nighswander-Rempel, S. P., R. A. Shaw, V. V. Kupriyanov, J. Rendell, B. Xiang, and H. H. Mantsch. Mapping tissue oxygenation in the beating heart with near-infrared spectroscopic imaging. Vib. Spectrosc. 1:85–89, 2003.
Nighswander-Rempel, S. P., R. A. Shaw, J. R. Mansfield, M. Hewko, V. V. Kupriyanov, and H. H. Mantsch. Regional variations in myocardial tissue oxygenation mapped by near-infrared spectroscopic imaging. J. Mol. Cell Cardiol. 9:1195–1203, 2002.
Nishioka, N. S., and M.-A. Mycek. Initial experience with a real-time video processor for enhancing endoscopic image contrast. Gastrointest. Endosc. 1:62–66, 1998.
Okatani, T., and K. Deguchi. Shape Reconstruction from an Endoscope Image by Shape from Shading Technique for a Point Light Source at the Projection Centre. Comput. Vis. Image Underst. 2:119–131, 1997.
Ortmaier, T., M. Gröger, D. H. Boehm, V. Falk, and G. Hirzinger. Motion estimation in beating heart surgery. IEEE Trans. Biomed. Eng. 10:1729–1740, 2005.
Ortmaier, T., M. Groger, and G. Hirzinger. Robust motion estimation in robotic surgery on the beating heart. In: Proceedings of Computer Assisted Radiology and Surgery, 2002.
Penne, J., K. Holler, M. Sturmer, T. Schrauder, A. Schneider, R. Engelbrecht, H. Feusner, B. Schmauss, and J. Hornegger. Time-of-Flight 3-D Endoscopy. In: Proceedings of the 12th International Conference on Medical Image Computing and Computer-Assisted Intervention: Part I, pp. 467–474, 2009.
Peters, T. M. Image-guidance for surgical procedures. Phys. Med. Biol. 51:R505–R540, 2006.
Pollefeys, M., L. V. Gool, and M. Proesmans. Stratified Self-Calibration from Image Sequences with Variable Focal Lengths. In: European Conference on Computer Vision, pp. 31–42, 1996.
Prados, E., and O. Faugeras. Shape From Shading: A Well Posed Problem? In: IEEE Conference on Computer Vision and Pattern Recognition, pp. 870–877, 2005.
Pratt, P., D. Stoyanov, M. Visentini-Scarzanella, and G.-Z. Yang. Dynamic guidance for robotic surgery using image- constrained biomechanical models. In: Proceedings of the 13th International Conference on Medical image Computing and Computer-Assisted Intervention: Part I, pp. 77–85, 2010.
Qiu, L., D. K. Pleskow, R. Chuttani, E. Vitkin, J. Leyden, N. Ozden, S. Itani, L. Guo, A. Sacks, J. D. Goldsmith, M. D. Modell, E. B. Hanlon, I. Itzkan, and L. T. Perelman. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett’s esophagus. Nat. Med. 5:603–606, 2010.
Rashid, H. U., and P. Burger. Differential algorithm for the determination of shape from shading using a point light source. Image Vis. Comput. 2:119–127, 1992.
Rattner, D., and A. Kalloo. ASGE/SAGES Working group on natural orifice translumenal endoscopic surgery. Surg. Endosc. 20:329–333, 2006.
Ren, J., J. Wu, E. J. McDowell, C. Yang, et al. Manual-scanning optical coherence tomography probe based on position tracking. Opt. Lett. 21:3400–3402, 2009.
Richa, R., M. Balicki, E. Meisner, R. Sznitman, R. Taylor, and G. Hager. Visual Tracking of Surgical Tools for Proximity Detection in Retinal Surgery. In: Information Processing in Computer-Assisted Interventions, pp. 55–66, 2011.
Richa, R., A. Bó, and P. Poignet. Robust 3D Visual Tracking for Robotic-Assisted Cardiac Interventions. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 267–274, 2010.
Richa, R., A. P. L. Bó, and P. Poignet. Towards robust 3D visual tracking for motion compensation in beating heart surgery. Med. Image Anal. 3:302–315, 2011.
Richa, R., P. Poignet, and C. Liu. Efficient 3D Tracking for Motion Compensation in Beating Heart Surgery. In: International Conference on Medical Image Computing and Computer Assisted Intervention, 2008.
Röhl, S., S. Bodenstedt, S. Suwelack, H. Kenngott, B. Mueller-Stich, R. Dillmanna, and S. Speidel. Real-Time Surface Reconstruction from Stereo Endoscopic Images for Intraoperative Registration. In: SPIE Medical Imaging, 2011.
Saint-Pierre, C.-A., J. Boisvert, G. Grimard, and F. Cheriet. Detection and correction of specular reflections for automatic surgical tool segmentation in thoracoscopic images. Mach. Vis. Appl. 1:171–180, 2011.
Scharstein, D., and R. Szeliski. A taxonomy and evaluation of dense two-frame stereo correspondence algorithms. Int. J. Comput. Vis. 1(2/3):7–42, 2002.
Seshamani, S. Direct global adjustment methods for endoscopic mosaicking. Proc. SPIE 1:72611D, 2009.
Seshamani, S., W. Lau, and G. Hager. Real-Time Endoscopic Mosaicking. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006, pp. 355–363, 2006.
Sorg, B. S., B. J. Moeller, Y. Cao, and M. W. Dewhirst. Hyperspectral imaging of hemoglobin saturation in tumor microvasculature and tumor hypoxia development. J. Biomed. Opt. 10:044004, 2005.
Srivastava, S., J. J. Rodriguez, A. R. Rouse, M. A. Brewer and A. F. Gmitro. Analysis of confocal microendoscope images for automatic detection of ovarian cancer. In: IEEE International Conference on Image Processing, 2005 (ICIP 2005), pp. I-1113–I-1116, 2005.
Stoyanov, D. Camera Calibration Tools. http://www.cs.ucl.ac.uk/staff/Dan.Stoyanov/calib/. 2011.
Stoyanov, D., A. Darzi, and G.-Z. Yang. Dense 3D Depth Recovery for Soft Tissue Deformation During Robotically Assisted Laparoscopic Surgery. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 41–48, 2004.
Stoyanov, D., A. Darzi, and G.-Z. Yang. Laparoscope Self-calibration for Robotic Assisted Minimally Invasive Surgery. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 114–121, 2005.
Stoyanov, D., D. Elson, and G.-Z. Yang. Illumination position estimation for 3D soft-tissue reconstruction in robotic minimally invasive surgery. Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, pp. 2628–2633, 2009.
Stoyanov, D., M. Lerotic, G. Mylonas, A. J. Chun, and G.-Z. Yang. Intra-operative Visualizations: Perceptual Fidelity and Human Factors. IEEE/OSA J. Display Technol. 4:491–501, 2008.
Stoyanov, D., G. P. Mylonas, F. Deligianni, A. Darzi, and G.-Z. Yang. Soft-tissue Motion Tracking and Structure Estimation for Robotic Assisted MIS Procedures. In: International Conference on Medical Image Computing and Computer Assisted Intervention, pp. 139–146, 2005.
Stoyanov, D., M. Scarzanella, P. Pratt, and G.-Z. Yang. Real-Time Stereo Reconstruction in Robotically Assisted Minimally Invasive Surgery. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010, pp. 275–282, 2010.
Stoyanov, D., and G.-Z. Yang. Removing specular reflection components for robotic assisted laparoscopic surgery. In: IEEE International Conference on Image Processing, 2005 (ICIP 2005), pp. III-632–III-635, 2005.
Stoyanov, D., and G.-Z. Yang. Soft tissue deformation tracking for robotic assisted minimally invasive surgery. In: 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2009), pp. 254–257, 2009.
Strobl, K. H., W. Sepp, S. Fuchs, C. Paredes, and K. Arbter. DLR CalLab CalDe: The DLR Camera Calibration Toolbox. http://www.robotic.dlr.de/callab/. 2011.
Suter, M. J., B. E. Bouma, and G. J. Tearney. High-Resolution Optical Coherence Tomography Imaging in Gastroenterology. In: Advances in Optical Imaging for Clinical Medicine, pp. 187–204, 2011.
Sznitman, R., S. Billings, D. Rother, D. Mirota, Y. Yang, J. Handa, P. Gehlbach, J. U. Kang, G. D. Hager, and R. Taylor. Active Multispectral Illumination and Image Fusion for Retinal Microsurgery. In: Proceedings of the First international Conference on Information Processing in Computer-Assisted Interventions, pp. 12–22, 2010.
Talamini, M. A., S. Chapman, S. Horgan, and W. S. Melvin. A prospective analysis of 211 robotic-assisted surgical procedures. Surg. Endosc. 10:1521–1524, 2003.
Tankus, A., N. Sochen, and Y. Yeshurun. Perspective Shape-from-Shading via Fast Marching. In: IEEE Computer Society Conference on Computer Vision and Pattern Recognition, 2004.
Taylor, R. H., and D. Stoianovici. Medical robotics in computer-integrated surgery. IEEE Trans. Robot. Autom. 5:765–781, 2003.
Tearney, G. J., S. A. Boppart, B. E. Bouma, M. E. Brezinski, N. J. Weissman, J. F. Southern, and J. G. Fujimoto. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt. Lett. 7:543–545, 1996.
Tearney, G. J., M. E. Brezinski, B. E. Bouma, S. A. Boppart, C. Pitris, J. F. Southern, and J. G. Fujimoto. In vivo endoscopic optical biopsy with optical coherence tomography. Science 5321:2037–2039, 1997.
Tonet, O., R. U. Thoranaghatte, G. Megali, and P. Dario. Tracking endoscopic instruments without a localizer: A shape-analysis-based approach. Comput. Aided Surg. 1:35–42, 2007.
Tsai, R. Y. An Efficient and Accurate Camera Calibration Technique for 3D Machine Vision. In: IEEE Computer Society Conference on Computer Vision and Pattern Recognition, pp. 364–374, 1986.
Uecker, D. R., Y. F. Wang, C. Lee, and Y. Wang. Laboratory investigation: automated instrument tracking in robotically assisted laparoscopic surgery. Comput. Aided Surg. 6:308–325, 1995.
Vercauteren, T., A. Perchant, X. Pennec, and N. Ayache. Mosaicing of Confocal Microscopic In Vivo Soft Tissue Video Sequences. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI 2005, pp. 753–760, 2005.
Voros, S., G. P. Haber, J. F. Menudet, J. A. Long, and P. Cinquin. ViKY Robotic scope holder: initial clinical experience and preliminary results using instrument tracking. IEEE/ASME Trans. Mechatron. 6:879–886, 2010.
Wang, P., S. M. Krishnan, Y. Huang, and N. Srinivasan. An adaptive segmentation technique for clinical endoscopic image processing. In: Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society EMBS/BMES Conference, Engineering in Medicine and Biology, Vol. 2, pp. 1084–1085, 2002.
Warren, A., P. Mountney, D. Noonan, and G.-Z. Yang. Horizon Stabilized—Dynamic View Expansion for Robotic Assisted Surgery (HS-DVE). In: International Journal of Computer Assisted Radiology and Surgery, pp. 1–8, 2011.
West, J. B., and C. R. Maurer, Jr. Designing optically tracked instruments for image-guided surgery. IEEE Trans. Med. Imaging 5:533–545, 2004.
Wu, J., M. Conry, C. Gu, F. Wang, Z. Yaqoob, C. Yang, et al. Paired-angle-rotation scanning optical coherence tomography forward-imaging probe. Opt. Lett. 9:1265–1267, 2006.
Wu, C., B. Jaramaz, and S. G. Narasimhan. A full geometric and photometric calibration method for oblique-viewing endoscopes. Comput. Aided Surg. 1–3:19–31, 2010.
Wu, C., S. Narasimhan, and B. Jaramaz. A Multi-Image Shape-from-Shading Framework for Near-Lighting Perspective Endoscopes. Int. J. Comput. Vis. 2:211–228, 2010.
Zhang, Z. A flexible new technique for camera calibration. IEEE Trans. Pattern Anal. Mach. Intell. 11:1330–1334, 2000.
Zhang, G., J. He, and X. Li. 3D vision inspection for internal surface based on circle structured light. Sens. Actuators A Phys. 1:68–75, 2005.
Zitová, B., and J. Flusser. Image registration methods: a survey. Image Vis. Comput. 11:977–1000, 2003.
Acknowledgments
The insightful and constructive comments of the anonymous reviewers were very helpful for improving this article. The work was carried out with the financial support of a Royal Academy of Engineering/EPSRC Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Daniel Elson oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Stoyanov, D. Surgical Vision. Ann Biomed Eng 40, 332–345 (2012). https://doi.org/10.1007/s10439-011-0441-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0441-z