Abstract
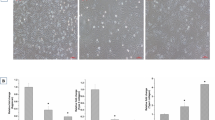
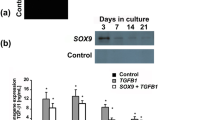
Autologous chondrocytes remain one of the most preferable candidates among various therapeutic cell species because of their high efficacy, despite remarkable progress in discovery and development of therapeutic cells for cartilage regenerative medicine to date. However, the essential process of cell expansion via repeated monolayer sub-cultures inevitably induces chondrocytic dedifferentiation. In this study, we aimed to achieve and enhance redifferentiation of dedifferentiated chondrocytes with dual genes of transforming growth factor (TGF)-β3 and short hairpin RNA (shRNA) that restore chondrocytic phenotype and silence fibrous collagen type I (Col I), respectively. It was hypothesized that gene delivery of the two targets would promote chondrogenesis in chondrocytes, and meanwhile inhibit the expression of the undesired Col I. Three types of recombinant adenoviruses were constructed. Two of them were of single-function vectors with the ability to express either TGF-β3 (Ad-TGFβ3) or shRNA (specific for Col I, Ad-shRNA); the third type was of double-function vectors that encode both TGF-β3 and anti-Col I shRNA (Ad-double). We infected the dedifferentiated chondrocytes with Ad-double, or co-transduced them with Ad-TGFβ3 and Ad-shRNA at the same time (designated as Ad-combination). Data from real-time RT-PCR and histological staining suggested a restoration in the expression of cartilage-specific genes including aggrecan, type II collagen, and cartilage oligomeric matrix protein (COMP); while a significant down-regulation of Col I expression was observed in groups treated with Ad-double and Ad-combination compared to other control groups. These results demonstrated that, by genetic modification, dedifferentiated chondrocytes managed to redifferentiate back to chondrocytic phenotype, which may greatly facilitate cartilage regenerative medicine by providing sufficient number of competent therapeutic cells.





Similar content being viewed by others
References
Benya, P. D., S. R. Padilla, and M. E. Nimni. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15(4):1313–1321, 1978.
Benya, P. D., and J. D. Shaffer. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30(1):215–224, 1982.
Bonaventure, J., N. Kadhom, L. Cohen-Solal, K. H. Ng, J. Bourguignon, C. Lasselin, and P. Freisinger. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp. Cell Res. 212(1):97–104, 1994.
Brittberg, M., A. Lindahl, A. Nilsson, C. Ohlsson, O. Isaksson, and L. Peterson. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331(14):889–895, 1994.
Buckwalter, J. A., and H. J. Mankin. Articular cartilage repair and transplantation. Arthritis Rheum. 41(8):1331–1342, 1998.
Chaipinyo, K., B. W. Oakes, and M. P. Van Damme. The use of debrided human articular cartilage for autologous chondrocyte implantation: maintenance of chondrocyte differentiation and proliferation in type I collagen gels. J. Orthop. Res. 22(2):446–455, 2004.
Chung, C., and J. A. Burdick. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60(2):243–262, 2008.
Darling, E. M., and K. A. Athanasiou. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23(2):425–432, 2005.
Enobakhare, B. O., D. L. Bader, and D. A. Lee. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal. Biochem. 243(1):189–191, 1996.
Hao, J., R. R. Varshney, and D. A. Wang. TGF-beta3: a promising growth factor in engineered organogenesis. Expert Opin. Biol. Ther. 8(10):1485–1493, 2008.
Hao, J., R. R. Varshney, and D. A. Wang. Engineering osteogenesis and chondrogenesis with gene-enhanced therapeutic cells. Curr. Opin. Mol. Ther. 11(4):404–410, 2009.
Hao, J., Y. Yao, R. R. Varshney, L. Wang, C. Prakash, H. Li, and D. A. Wang. Gene transfer and living release of transforming growth factor-beta3 for cartilage tissue engineering applications. Tissue Eng. Part C Methods 14(4):273–280, 2008.
Huch, K., J. Stove, W. Puhl, and K. P. Gunther. Review and comparison of culture-techniques for articular chondrocytes. Z. Orthop. Ihre. Grenzgeb. 140(2):145–152, 2002.
Kim, Y. J., R. L. Sah, J. Y. Doong, and A. J. Grodzinsky. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 174(1):168–176, 1988.
Lin, L., C. Zhou, X. Wei, Y. Hou, L. Zhao, X. Fu, J. Zhang, and C. Yu. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 58(4):1067–1075, 2008.
Malemud, C. J., S. Stevenson, F. Mehraban, R. S. Papay, A. F. Purchio, and V. M. Goldberg. The proteoglycan synthesis repertoire of rabbit chondrocytes maintained in type II collagen gels. Osteoarthr Cartil 2(1):29–41, 1994.
Marlovits, S., P. Zeller, P. Singer, C. Resinger, and V. Vecsei. Cartilage repair: generations of autologous chondrocyte transplantation. Eur. J. Radiol. 57(1):24–31, 2006.
Miyanishi, K., M. C. Trindade, D. P. Lindsey, G. S. Beaupre, D. R. Carter, S. B. Goodman, D. J. Schurman, and R. L. Smith. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 12(8):2253–2262, 2006.
Morales, T. I., and A. B. Roberts. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J. Biol. Chem. 263(26):12828–12831, 1988.
Redini, F., M. Daireaux, A. Mauviel, P. Galera, G. Loyau, and J. P. Pujol. Characterization of proteoglycans synthesized by rabbit articular chondrocytes in response to transforming growth factor-beta (TGF-beta). Biochim. Biophys. Acta 1093(2–3):196–206, 1991.
Reginato, A. M., R. V. Iozzo, and S. A. Jimenez. Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum. 37(9):1338–1349, 1994.
Sellers, R. S., D. Peluso, and E. A. Morris. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 79(10):1452–1463, 1997.
Sung, L. Y., H. Y. Chiu, H. C. Chen, Y. L. Chen, C. K. Chuang, and Y. C. Hu. Baculovirus-mediated growth factor expression in dedifferentiated chondrocytes accelerates redifferentiation: effects of combinational transduction. Tissue Eng. Part A. 15(6):1353–1362, 2009.
Sung, L. Y., W. H. Lo, H. Y. Chiu, H. C. Chen, C. K. Chung, H. P. Lee, and Y. C. Hu. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials 28(23):3437–3447, 2007.
Ting, V., C. D. Sims, L. E. Brecht, J. G. McCarthy, A. K. Kasabian, P. R. Connelly, J. Elisseeff, G. K. Gittes, and M. T. Longaker. In vitro prefabrication of human cartilage shapes using fibrin glue and human chondrocytes. Ann. Plast. Surg. 40(4):413–420, 1998; discussion 420-1.
Walther, W., and U. Stein. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs 60(2):249–271, 2000.
Weng, H. H., and J. Fitzgerald. Current issues in joint replacement surgery. Curr. Opin. Rheumatol. 18(2):163–169, 2006.
Wu, T. L., and D. Zhou. Viral delivery for gene therapy against cell movement in cancer. Adv. Drug Deliv. Rev. 63(8):671–677, 2011.
Yao, Y., C. Wang, R. R. Varshney, and D. A. Wang. Antisense makes sense in engineered regenerative medicine. Pharm. Res. 26(2):263–275, 2009.
Yao, Y., F. Zhang, R. Zhou, M. Li, and D. A. Wang. Continuous supply of TGFbeta3 via adenoviral vector promotes type I collagen and viability of fibroblasts in alginate hydrogel. J. Tissue Eng. Regen. Med. 4(7):497–504, 2010.
Yao, Y., F. Zhang, R. Zhou, K. Su, J. Fan, and D. A. Wang. Effects of combinational adenoviral vector-mediated TGF beta 3 transgene and shRNA silencing type I collagen on articular chondrogenesis of synovium-derived mesenchymal stem cells. Biotechnol. Bioeng. 106(5):818–828, 2010.
Yun, K., and H. T. Moon. Inducing chondrogenic differentiation in injectable hydrogels embedded with rabbit chondrocytes and growth factor for neocartilage formation. J. Biosci. Bioeng. 105(2):122–126, 2008.
Zhang, F., Y. Yao, J. Hao, R. Zhou, C. Liu, Y. Gong, and D. A. Wang. A dual-functioning adenoviral vector encoding both transforming growth factor-beta3 and shRNA silencing type I collagen: construction and controlled release for chondrogenesis. J. Control Release 142(1):70–77, 2010.
Acknowledgments
This research was financially supported by AcRF Tier 1 Grant RG64/08, Ministry of Education (MoE), Singapore.
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael S. Detamore oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Yao, Y., Su, K. et al. Redifferentiation of Dedifferentiated Chondrocytes by Adenoviral Vector-Mediated TGF-β3 and Collagen-1 Silencing shRNA in 3D Culture. Ann Biomed Eng 39, 3042–3054 (2011). https://doi.org/10.1007/s10439-011-0398-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0398-y