Abstract
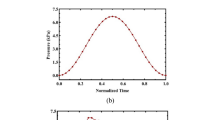
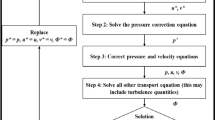
Locally disturbed flow has been suggested to play a (modulating) role in abdominal aortic aneurysm (AAA) formation, but no longitudinal studies have been performed yet due to (a.o.) a lack of human data prior to AAA formation. In this study we made use of recent advances in small animal imaging technology in order to set up entirely mouse-specific computational fluid dynamics (CFD) simulations of the abdominal aorta in an established ApoE −/− mouse model of AAA formation, combining (i) in vivo contrast-enhanced micro-CT scans (geometrical model) and (ii) in vivo high-frequency ultrasound scans (boundary conditions). Resulting areas of disturbed flow at baseline were compared to areas of AAA at end-stage. Qualitative results showed that AAA dimension is maximal in areas that are situated proximal to those areas that experience most disturbed flow in three out of four S developing an AAA. Although further quantitative analysis did not reveal any obvious relationship between areas that experience most disturbed flow and the end-stage AAA dimensions, we cannot exclude that hemodynamics play a role in the initial phases of AAA formation. Due to its mouse-specific and in vivo nature, the presented methodology can be used in future research to link detailed and animal-specific (baseline) hemodynamics to (end-stage) arterial disease in longitudinal studies in mice.






Similar content being viewed by others
References
Alexander, J. J. The pathobiology of aortic aneurysms. J. Surg. Res. 117:163–175, 2004.
Amirbekian, S., R. C. Long, M. A. Consolini, J. Suo, N. J. Willett, S. W. Fielden, D. P. Giddens, W. R. Taylor, and J. N. Oshinski. In vivo assessment of blood flow patterns in abdominal aorta of mice with MRI: implications for AAA localization. Am. J. Physiol. Heart Circ. Physiol. 297:H1290–H1295, 2009.
Antiga, L., and D. A. Steinman. http://www.vmtk.org.
Antiga, L., and D. A. Steinman. Robust and objective decomposition and mapping of bifurcating vessels. IEEE Trans. Med. Imaging 23:704–713, 2004.
Barisione, C., D. L. Rateri, J. J. Moorleghen, D. A. Howatt, and A. Daugherty. Angiotensin II infusion promotes rapid dilation of the abdominal aorta detected by noninvasive high frequency ultrasound. Arterioscler. Thromb. Vasc. Biol. 26:E73, 2006.
Biasetti, J., T. C. Gasser, M. Auer, U. Hedin, and F. Labruto. Hemodynamics of the normal aorta compared to fusiform and saccular abdominal aortic aneurysms with emphasis on a potential thrombus formation mechanism. Ann. Biomed. Eng. 38:380–390, 2010.
Boussel, L., V. Rayz, C. McCulloch, A. Martin, G. Acevedo-Bolton, M. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39:2997–3002, 2008.
Cheng, C., D. Tempel, R. van Haperen, A. van der Baan, F. Grosveld, M. J. A. P. Daemen, R. Krams, and R. de Crom. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753, 2006.
Daugherty, A., and L. Cassis. Angiotensin II and abdominal aortic aneurysms. Curr. Hypertens. Rep. 6:442–446, 2004.
Daugherty, A., and L. A. Cassis. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 24:429–434, 2004.
Daugherty, A., M. W. Manning, and L. A. Cassis. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105:1605–1612, 2000.
Daugherty, A., D. L. Rateri, I. F. Charo, A. P. Owens, D. A. Howatt, and L. A. Cassis. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE−/− mice. Clin. Sci. 118:681–689, 2010.
De Santis, G., P. Mortier, M. De Beule, P. Segers, P. Verdonck, and B. Verhegghe. Patient-specific computational fluid dynamics: structured mesh generation from coronary angiography. Med. Biol. Eng. Comput. 48:371–380, 2010.
Feintuch, A., P. Ruengsakulrach, A. Lin, J. Zhang, Y. Q. Zhou, J. Bishop, L. Davidson, D. Courtman, F. S. Foster, D. A. Steinman, R. M. Henkelman, and C. R. Ethier. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292:H884–H892, 2007.
Finol, E. A., and C. H. Amon. Blood flow in abdominal aortic aneurysms: pulsatile flow hemodynamics. J. Biomech. Eng. 123:474–484, 2001.
Finol, E. A., K. Keyhani, and C. H. Amon. The effect of asymmetry in abdominal aortic aneurysms under physiologically realistic pulsatile flow conditions. J. Biomech. Eng. 125:207–217, 2003.
Goergen, C. J., J. Azuma, K. N. Barr, L. Magdefessel, D. Y. Kallop, A. Gogineni, A. Grewall, R. M. Weimer, A. J. Connolly, R. L. Dalman, C. A. Taylor, P. S. Tsao, and J. M. Greve. Influences of aortic motion and curvature on vessel expansion in murine experimental aneurysms. Arterioscler. Thromb. Vasc. Biol. 31:270–279, 2011.
Goergen, C. J., K. N. Barr, D. T. Huynh, J. R. Eastham-Anderson, G. Choi, M. Hedehus, R. L. Dalman, A. J. Connolly, C. A. Taylor, P. S. Tsao, and J. M. Greve. In vivo quantification of murine aortic cyclic strain, motion, and curvature: implications for abdominal aortic aneurysm growth. J. Magn. Reson. Imaging 32:847–858, 2010.
Golledge, J., J. Muller, A. Daugherty, and P. Norman. Abdominal aortic aneurysm—pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 26:2605–2613, 2006.
Gordon, I. L., C. A. Kohl, M. Arefi, R. A. Complin, and M. Vulpe. Spinal cord injury increases the risk of abdominal aortic aneurysm. Am. Surg. 62:249–252, 1996.
Greve, J. M., A. S. Les, B. T. Tang, M. T. D. Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, and C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291:H1700–H1708, 2006.
Huo, Y. L., X. M. Guo, and G. S. Kassab. The flow field along the entire length of mouse aorta and primary branches. Ann. Biomed. Eng. 36:685–699, 2008.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation—positive correlation between plaque location and low and oscillating shear–stress. Arteriosclerosis 5:293–302, 1985.
Lee, S. W., L. Antiga, and D. A. Steinman. Correlations among indicators of disturbed flow at the normal carotid bifurcation. J. Biomech. Eng. Trans. ASME. 131, 2009.
Les, A. S., S. C. Shadden, C. A. Figueroa, J. M. Park, M. M. Tedesco, R. J. Herfkens, R. L. Dalman, and C. A. Taylor. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 38:1288–1313, 2010.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Mantha, A., C. Karmonik, G. Benndorf, C. Strother, and R. Metcalfe. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. Am. J. Neuroradiol. 27:1113–1118, 2006.
Meng, H., Z. Wang, Y. Hoi, L. Gao, E. Metaxa, D. D. Swartz, and J. Kolega. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38:1924–1931, 2007.
Moore, J. E., C. P. Xu, S. Glagov, C. K. Zarins, and D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta—oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110:225–240, 1994.
Patel, M. I., D. T. A. Hardman, and C. M. Fisher. Current views on the pathogenesis of abdominal aortic aneurysms. J. Am. Coll. Surg. 181:371–382, 1995.
Sakalihasan, N., R. Limet, and O. D. Defawe. Abdominal aortic aneurysm. Lancet 365:1577–1589, 2005.
Saraff, K., F. Babamusta, L. A. Cassis, and A. Daugherty. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23:1621–1626, 2003.
Shahcheraghi, N., H. A. Dwyer, A. Y. Cheer, A. I. Barakat, and T. Rutaganira. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J. Biomech. Eng. Trans. ASME 124:378–387, 2002.
Shimogonya, Y., T. Ishikawa, Y. Imai, N. Matsuki, and T. Yamaguchi. Can temporal fluctuation in spatial wall shear stress gradient initiate a cerebral aneurysm? A proposed novel hemodynamic index, the gradient oscillatory number (GON). J. Biomech. 42:550–554, 2009.
Shipkowitz, T., V. G. J. Rodgers, L. J. Frazin, and K. B. Chandran. Numerical study on the effect of secondary flow in the human aorta on local shear stresses in abdominal aortic branches. J. Biomech. 33:717–728, 2000.
Suo, J., D. E. Ferrara, D. Sorescu, R. E. Guldberg, W. R. Taylor, and D. P. Giddens. Hemodynamic shear stresses in mouse aortas—implications for atherogenesis. Arterioscler. Thromb. Vasc. Biol. 27:346–351, 2007.
Trachet, B., A. Swillens, D. Van Loo, C. Casteleyn, A. De Paepe, B. Loeys, and P. Segers. The influence of aortic dimensions on calculated wall shear stress in the mouse aortic arch. Comput. Methods Biomech. Biomed. Eng. 12:491–499, 2009.
Vandeghinste, B., B. Trachet, M. Renard, C. Casteleyn, S. Staelens, B. Loeys, P. Segers, and S. Vandenberghe. Replacing vascular corrosion casting by in vivo micro-CT imaging for building 3D cardiovascular models in mice. Mol. Imaging Biol. 13:78–86, 2011.
Verhegge, B. http://www.pyFormex.org.
Vollmar, J. F., P. Pauschinger, E. Paes, E. Henze, and A. Friesch. Aortic aneurysms as late sequelae of above-knee amputation. Lancet 2:834–835, 1989.
Yeung, J. J., H. J. Kim, T. A. Abbruzzese, I. E. Vignon-Clementel, M. T. Draney-Blomme, K. K. Yeung, I. Perkash, R. J. Herfkens, C. A. Taylor, and R. L. Dalman. Aortoiliac hemodynamic and morphologic adaptation to chronic spinal cord injury. J. Vasc. Surg. 44:1254–1265, 2006.
Acknowledgments
This research was funded by the Special Research Fund of the Ghent University (BOF10/GOA/005) and the Hercules Foundation (project AUGE/09/012). Bram Trachet is recipient of a research grant of the Flemish government agency for Innovation by Science and Technology (IWT). The authors wish to acknowledge the assistance of Philippe Joye and Scharon Bruneel from the Ghent University small animal imaging lab (INFINITY).
Conflicts of interest
There are no conflicts of interests to be declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joan Greve oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Trachet, B., Renard, M., De Santis, G. et al. An Integrated Framework to Quantitatively Link Mouse-Specific Hemodynamics to Aneurysm Formation in Angiotensin II-infused ApoE −/− mice. Ann Biomed Eng 39, 2430–2444 (2011). https://doi.org/10.1007/s10439-011-0330-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0330-5