Abstract
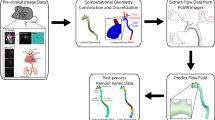
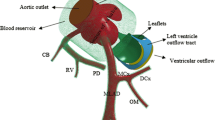
Following surgical induction of aortic valve regurgitation (AR), extensive atherosclerotic plaque development along the descending thoracic and abdominal aorta of Ldlr −/− mice has been reported, with distinct spatial distributions suggestive of a strong local hemodynamic influence. The objective of this study was to test, using image-based computational fluid dynamics (CFD), whether this is indeed the case. The lumen geometry was reconstructed from micro-CT scanning of a control Ldlr −/− mouse, and CFD simulations were carried out for both AR and control flow conditions derived from Doppler ultrasound measurements and literature data. Maps of time-averaged wall shear stress magnitude (TAWSS), oscillatory shear index (OSI) and relative residence time (RRT) were compared against the spatial distributions of plaque stained with oil red O, previously acquired in a group of AR and control mice. Maps of OSI and RRT were found to be consistent with plaque distributions in the AR mice and the absence of plaque in the control mice. TAWSS was uniformly lower under control vs. AR flow conditions, suggesting that levels (>100 dyn/cm2) exceeded those required to alone induce a pro-atherogenic response. Simulations of a straightened CFD model confirmed the importance of anatomical curvature for explaining the spatial distribution of lesions in the AR mice. In summary, oscillatory and retrograde flow induced in the AR mice, without concomitant low shear, may exacerbate or accelerate lesion formation, but the distinct anatomical curvature of the mouse aorta is responsible for the spatial distribution of lesions.






Similar content being viewed by others
References
Antiga, L., M. Piccinelli, L. Botti, B. Ene-Iordache, A. Remuzzi, and D. A. Steinman. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 46:1097–1112, 2008.
Berk, B. C., J. I. Abe, W. Min, J. Surapisitchat, and C. Yan. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann. N. Y. Acad. Sci. 947:93–109, 2001; (discussion 109–111).
Caro, C. G. Discovery of the role of wall shear in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29:158–161, 2009.
Cheng, C., F. Helderman, D. Tempel, D. Segers, B. Hierck, R. Poelmann, A. van Tol, D. J. Duncker, D. Robbers-Visser, N. T. Ursem, R. van Haperen, J. J. Wentzel, F. Gijsen, A. F. van der Steen, R. de Crom, and R. Krams. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195:225–235, 2007.
Cheng, C., D. Tempel, R. van Haperen, A. van der Baan, F. Grosveld, M. J. Daemen, R. Krams, and R. de Crom. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753, 2006.
Ethier, C. R., S. Prakash, D. A. Steinman, R. L. Leask, G. G. Couch, and M. Ojha. Steady flow separation patterns in a 45 degree junction. J. Fluid Mech. 411:1–38, 2000.
Feintuch, A., P. Ruengsakulrach, A. Lin, J. Zhang, Y. Q. Zhou, J. Bishop, L. Davidson, D. Courtman, F. S. Foster, D. A. Steinman, R. M. Henkelman, and C. R. Ethier. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292:H884–H892, 2007.
Greve, J. M., A. S. Les, B. T. Tang, M. T. Draney Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, and C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291:H1700–H1708, 2006.
Himburg, H. A., D. M. Grzybowski, A. L. Hazel, J. A. LaMack, X. M. Li, and M. H. Friedman. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am. J. Physiol. Heart Circ. Physiol. 286:H1916–H1922, 2004.
Huo, Y., X. Guo, and G. S. Kassab. The flow field along the entire length of mouse aorta and primary branches. Ann. Biomed. Eng. 36:685–699, 2008.
Ishibashi, S., M. S. Brown, J. L. Goldstein, R. D. Gerard, R. E. Hammer, and J. Herz. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92:883–893, 1993.
Jin, S., J. Oshinski, and D. P. Giddens. Effects of wall motion and compliance on flow patterns in the ascending aorta. J. Biomech. Eng. 125:347–354, 2003.
Knight, J., U. Olgac, S. C. Saur, D. Poulikakos, W. Marshall, Jr., P. C. Cattin, H. Alkadhi, and V. Kurtcuoglu. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human right coronary arteries. Atherosclerosis 211:445–450, 2010.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5:293–302, 1985.
Lee, S. W., L. Antiga, and D. A. Steinman. Correlations among indicators of disturbed flow at the normal carotid bifurcation. J. Biomech. Eng. 131:061013, 2009.
Libby, P. Inflammation in atherosclerosis. Nature 420:868–874, 2002.
Libby, P., P. M. Ridker, and A. Maseri. Inflammation and atherosclerosis. Circulation 105:1135–1143, 2002.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Minev, P. D., and C. R. Ethier. A characteristic/finite element algorithm for the 3-D Navier–Stokes equations using unstructured grids. Comput. Methods Appl. Mech. Eng. 178:39–50, 1998.
Moore, J. E., Jr., S. E. Maier, D. N. Ku, and P. Boesiger. Hemodynamics in the abdominal aorta: a comparison of in vitro and in vivo measurements. J. Appl. Physiol. 76:1520–1527, 1994.
Plump, A. S., J. D. Smith, T. Hayek, K. Aalto-Setala, A. Walsh, J. G. Verstuyft, E. M. Rubin, and J. L. Breslow. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71:343–353, 1992.
Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340:115–126, 1999.
Suo, J., D. E. Ferrara, D. Sorescu, R. E. Guldberg, W. R. Taylor, and D. P. Giddens. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler. Thromb. Vasc. Biol. 27:346–351, 2007.
Thijssen, D. H., E. A. Dawson, T. M. Tinken, N. T. Cable, and D. J. Green. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53:986–992, 2009.
Tozer, E. C., and T. E. Carew. Residence time of low-density lipoprotein in the normal and atherosclerotic rabbit aorta. Circ. Res. 80:208–218, 1997.
Trachet, B., A. Swillens, D. Van Loo, C. Casteleyn, A. De Paepe, B. Loeys, and P. Segers. The influence of aortic dimensions on calculated wall shear stress in the mouse aortic arch. Comput. Methods Biomech. Biomed. Eng. 12:491–499, 2009.
Weinberg, P. D., and C. Ross Ethier. Twenty-fold difference in hemodynamic wall shear stress between murine and human aortas. J. Biomech. 40:1594–1598, 2007.
Willett, N. J., R. C. Long, Jr., K. Maiellaro-Rafferty, R. L. Sutliff, R. Shafer, J. N. Oshinski, D. P. Giddens, R. E. Guldberg, and W. R. Taylor. An in vivo murine model of low-magnitude oscillatory wall shear stress to address the molecular mechanisms of mechanotransduction—brief report. Arterioscler. Thromb. Vasc. Biol. 30:2099–2102, 2010.
Williams, K. J., and I. Tabas. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15:551–561, 1995.
Williams, K. J., and I. Tabas. Atherosclerosis and inflammation. Science 297:521–522, 2002.
Zhou, Y. Q., L. Davidson, R. M. Henkelman, B. J. Nieman, F. S. Foster, L. X. Yu, and X. J. Chen. Ultrasound-guided left-ventricular catheterization: a novel method of whole mouse perfusion for microimaging. Lab Invest. 84:385–389, 2004.
Zhou, Y. Q., F. S. Foster, B. J. Nieman, L. Davidson, X. J. Chen, and R. M. Henkelman. Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol. Genomics 18:232–244, 2004.
Zhou, Y. Q., S. N. Zhu, F. S. Foster, M. I. Cybulsky, and R. M. Henkelman. Aortic regurgitation dramatically alters the distribution of atherosclerotic lesions and enhances atherogenesis in mice. Arterioscler. Thromb. Vasc. Biol. 30:1181–1188, 2010.
Acknowledgments
The authors thank Lisa Yu for the micro-CT scanning. This work is part of the Mouse Imaging Centre at the Hospital for Sick Children and the University of Toronto. The infrastructure was funded by the Canada Foundation for Innovation and Ontario Innovation Trust. The research was funded by a CIHR Grant (102590), and the Heart and Stroke Foundation of Ontario (Grants T6107 and T6060). R.M.H. holds a Canada Research Chair. Y.H. and D.A.S. acknowledge the support of a Heart & Stroke Foundation Research Fellowship Award and Career Investigator Award, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joan Greve oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Hoi, Y., Zhou, YQ., Zhang, X. et al. Correlation Between Local Hemodynamics and Lesion Distribution in a Novel Aortic Regurgitation Murine Model of Atherosclerosis. Ann Biomed Eng 39, 1414–1422 (2011). https://doi.org/10.1007/s10439-011-0255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0255-z