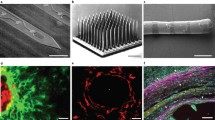
Abstract
Neural prosthetic devices hold the potential to be used in the treatment of a variety of neurological disorders. However, their long-term clinical success is currently limited by the ability to achieve stable interfaces between devices and the CNS. Immunohistochemical analysis has shown that cellular responses occur in tissue surrounding implanted devices. These cellular responses have been correlated with the impedance measured from device electrodes, leading to the hypothesis that a possible mechanism resulting in inconsistent device performance is the formation of an electrically insulating glial sheath at the implantation site. However, little is known about what cellular and tissue changes affect impedance values and thus contribute to the decreases in electrode performance. We have designed an in vitro system in which cell conditions can be varied within an artificial tissue matrix surrounding a neural prosthetic device. In this study, high-density cultures of glial cells were analyzed by immunohistochemical methods and impedance spectroscopy. Astrocytes and microglia were cultured at various ratios within the matrix surrounding the probes, and were observed over a period of 2 weeks. Cell seeding conditions and confocal images were compared to impedance data to enable the effects of glial cell type on electrode impedance to be determined.








Similar content being viewed by others
References
Andrade, D. M., et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology 66(10):1571–1573, 2006.
Banker, G., and K. Goslin. Culturing Nerve Cells. Cambridge: MIT Press, 1998.
Bard, A. J., and L. R. Faulkner. Electrochemical Methods: Fundamentals and Applications. New York: John Wiley & Sons, 2001.
Biran, R., D. C. Martin, and P. A. Tresco. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195(1):115–126, 2005.
Biran, R., D. C. Martin, and P. A. Tresco. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 82(1):169–178, 2007.
Bjornsson, C. S., et al. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 3(3):196–207, 2006.
Bjornsson, C. S., et al. Associative image analysis: a method for automated quantification of 3D multi-parameter images of brain tissue. J. Neurosci. Methods 170(1):165–178, 2008.
Butson, C. R., and C. C. McIntyre. Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clin. Neurophysiol. 116(10):2490–2500, 2005.
Butson, C. R., et al. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34(2):661–670, 2007.
Denes, A., et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. 27(12):1941–1953, 2007.
Dhoot, N. O., et al. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A 71(2):191–200, 2004.
Drury, J. L., R. G. Dennis, and D. J. Mooney. The tensile properties of alginate hydrogels. Biomaterials 25(16):3187–3199, 2004.
Dutcher, S. A., et al. Patterns of immediate early gene mRNA expression following rodent and human traumatic brain injury. Neurol. Res. 21(3):234–242, 1999.
Eiselt, P., et al. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials 21(19):1921–1927, 2000.
Frampton, J. P., M. L. Shuler, M. R. Hynd, and W. Shain. Fabrication and optimization of alginate hydrogels for neural cell culture, 2009 (In Submission).
Frampton, J. P., et al. Three-dimensional hydrogel cultures for modeling changes in tissue impedance around microfabricated neural probes. J. Neural Eng. 4(4):399–409, 2007.
Franks, W., et al. Impedance characterization and modeling of electrodes for biomedical applications. IEEE Trans. Biomed. Eng. 52(7):1295–1302, 2005.
Griffith, R. W., and D. R. Humphrey. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci. Lett. 406(1–2):81–86, 2006.
Haynes, S. E., et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9(12):1512–1519, 2006.
Kong, H. J., M. K. Smith, and D. J. Mooney. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 24(22):4023–4029, 2003.
Kong, H. J., et al. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 5(5):1720–1727, 2004.
Koshinaga, M., et al. Rapid microglial activation induced by traumatic brain injury is independent of blood brain barrier disruption. Histol. Histopathol. 22(2):129–135, 2007.
Limousin, P., and I. Martinez-Torres. Deep brain stimulation for Parkinson’s disease. Neurotherapeutics 5(2):309–319, 2008.
Liu, X., et al. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehabil. Eng. 7(3):315–326, 1999.
Martin, D. L., and W. Shain. High affinity transport of taurine and beta-alanine and low affinity transport of gamma-aminobutyric acid by a single transport system in cultured glioma cells. J. Biol. Chem. 254(15):7076–7084, 1979.
Merrill, D. R., and P. A. Tresco. Impedance characterization of microarray recording electrodes in vitro. IEEE Trans. Biomed. Eng. 52(11):1960–1965, 2005.
Normann, R. A. Technology insight: future neuroprosthetic therapies for disorders of the nervous system. Nat. Clin. Pract. Neurol. 3(8):444–452, 2007.
Polikov, V. S., P. A. Tresco, and W. M. Reichert. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148(1):1–18, 2005.
Preston, E., J. Webster, and D. Small. Characteristics of sustained blood–brain barrier opening and tissue injury in a model for focal trauma in the rat. J. Neurotrauma 18(1):83–92, 2001.
Retterer, S. T., et al. Model neural prostheses with integrated microfluidics: a potential intervention strategy for controlling reactive cell and tissue responses. IEEE Trans. Biomed. Eng. 51(11):2063–2073, 2004.
Rowley, J. A., G. Madlambayan, and D. J. Mooney. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20(1):45–53, 1999.
Schalk, G., et al. Two-dimensional movement control using electrocorticographic signals in humans. J. Neural Eng. 5(1):75–84, 2008.
Schiff, N. D., et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 448(7153):600–603, 2007.
Schwab, J. M., K. Seid, and H. J. Schluesener. Traumatic brain injury induces prolonged accumulation of cyclooxygenase-1 expressing microglia/brain macrophages in rats. J. Neurotrauma 18(9):881–890, 2001.
Schwartz, A. B. Cortical neural prosthetics. Annu. Rev. Neurosci. 27:487–507, 2004.
Seymour, J. P., and D. R. Kipke. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 28(25):3594–3607, 2007.
Siddiqui, M. S., et al. Deep brain stimulation: treating neurological and psychiatric disorders by modulating brain activity. NeuroRehabilitation 23(1):105–113, 2008.
Spataro, L., et al. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp. Neurol. 194(2):289–300, 2005.
Szarowski, D. H., et al. Brain responses to micro-machined silicon devices. Brain Res. 983(1–2):23–35, 2003.
Turner, J. N., et al. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 156(1):33–49, 1999.
Williams, J. C., et al. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 4(4):410–423, 2007.
Winter, J. O., S. F. Cogan, and J. F. Rizzo, 3rd. Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J. Biomed. Mater. Res. B Appl. Biomater 81(2):551–563, 2007.
Wolpaw, J. R., and D. J. McFarland. Control of a two-dimensional movement signal by a noninvasive brain–computer interface in humans. Proc. Natl Acad. Sci. USA 101(51):17849–17854, 2004.
Acknowledgments
The authors would like to acknowledge the Wadsworth Center Advanced Light Microscopy and Image Analysis Core and its director, Richard Cole. This work was supported in part by the Nanobiotechnology Center (NBTC) an STC program of the NSF under agreement number ECS-9876771, by NIBIB (R21R21EB007782), and by NIH (NS R01-04488145).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor John A. White oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Alginate and Probe Setup. NeuroNexus probes/PCBs were mounted on glass frames. Teflon chambers were attached to the glass frames using PDMS, providing a well to contain cell culture medium. Diagrams illustrate the approximate position of the probe (containing electrodes) relative to the glass frame and the alginate matrix. Supplementary material 1 (TIFF 77520 kb)
Rights and permissions
About this article
Cite this article
Frampton, J.P., Hynd, M.R., Shuler, M.L. et al. Effects of Glial Cells on Electrode Impedance Recorded from Neural Prosthetic Devices In Vitro . Ann Biomed Eng 38, 1031–1047 (2010). https://doi.org/10.1007/s10439-010-9911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9911-y