Abstract
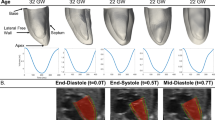
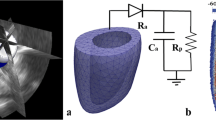
Knowledge of normal fetal heart (FH) performance and development is crucial for evaluating and understanding how various congenital heart lesions may modify heart contractility during the gestational period. However, since biomechanical models of FH are still lacking, structural approaches proposed to describe the mechanical behavior of the adult human heart cannot be used to model the evolution of the FH. In this paper, a finite element model of the healthy FH wall is developed to quantify its mechanical properties during the gestational period. An idealized thick-walled ellipsoidal shape was used to model the left ventricle (LV). The diastolic LV geometry was reconstructed from in vivo ultrasound measurements performed on 24 normal FHs between 20 and 37 weeks of gestation. An anisotropic hyperelastic constitutive law describing the mechanical properties of the passive and active myocardium was used. The evolution of the mechanical properties of the normal LV myocardium during fetal growth was obtained by successfully fitting the ejection fraction predicted by the model to in vivo measurements. We found that only the active tension varies significantly during the gestational period, increasing linearly from 20 kPa (at 20 weeks) to 40 kPa (at 37 weeks of gestation). We propose a possible explanation of the increasing force-generating ability of the myocardial tissue during fetal heart development based on a combination of myocyte enlargement, differentiation, and proliferation kinetics.











Similar content being viewed by others
References
Bishop, S. P., and P. Hine. Cardiac muscle cytoplasmic and nuclear development during canine neonatal growth. Recent Adv. Stud. Card. Struct. Metab. 8:77–98, 1975.
Bourdarias, C., S. Gerbi, and J. Ohayon. A three dimensional finite element method for biological active soft tissue. Formulation in cylindrical polar coordinates. ESAIM: Math. Model Numer. Anal. 37:725–739, 2003.
Chadwick, R. Mechanics of the left ventricle. Biophys. J. 39:279–288, 1982.
de Almeida, A., T. McQuinn, and D. Sedmera. Normal and hypoplastic fetal chick left ventricle increased ventricular preload is compensated by myocyte proliferation. Circ. Res. 100:1363–1370, 2007.
Fiorina, P., D. Corradi, S. Pinelli, R. Maestri, C. Lagrasta, M. Buscaglia, A. Davalli, F. Folli, and E. Astorri. Apoptotic/mytogenic pathways during human heart development. Int. J. Cardiol. 96:409–417, 2004.
Friedman, W. The intrinsic physiologic properties of the developing heart. Prog. Cardiovasc. Dis. 15:87–111, 1972.
Fung, Y. C. Biomechanics. Mechanical Properties of Living Tissues. New York: Springer, 1993.
Garcia, R. H., M. P. Cabeza, A. Gallo, L. Palacios, and R. P. Laguens. DNA content and expression of cell cycle proteins in caterpillar nuclei from fetal human cardiac myocytes. Virchows. Arch. 440:45–49, 2002.
Garcia-Palomares, U. M., and J. F. Rodríguez. New sequential and parallel derivative-free algorithms for unconstrained minimization. SIAM J. Optim. 13:79, 2006.
Garzón-Alvarado, D. A., J. M. García-Aznar, and M. Doblaré. A reaction–diffusion model for long bones growth. Biomechan. Model Mechanobiol. 8:381–395, 2009.
Guccione, J., A. McCulloch, and L. Waldman. Passive material properties of intact ventricular myocardium determined from a cylindrical model. ASME J. Biomech. Eng. 113:42–55, 1991.
Hibbit, Karlsson Sorensen, Inc. Abaqus User’s Guide, v.6.8. Pawtucket, RI, USA: HKS Inc., 2008.
Holzapfel, G. A. Nonlinear Solid Mechanics. New York: Wiley, 2000.
Hsieh, Y. Y., F. C. Chang, H. D. Tsai, and C. H. Tsai. Longitudinal survey of fetal ventricular ejection and shortening fraction throughout pregnancy. Ultrasound Obstet. Gynecol. 16:46–48, 2000.
Humphrey, J., and F. Yin. Constitutive relations and finite deformations of passive cardiac tissue. II: stress analysis in the left ventricle. Circ. Res. 65:805–817, 1989.
Hunter, P., A. D. McCulloch, and H. ter Keurs. Modelling the mechanical properties of cardiac muscle. Prog. Biophys. Mol. Biol. 69:289–331, 1998.
Hunter, P., A. J. Pullan, and B. H. Smaill. Modeling total heart function. Annu. Rev. Biomed. Eng. 5:147–177, 2003.
Huttenbach, Y., M. L. Ostrowski, D. Thaller, and H. S. Kim. Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc. Pathol. 10:119–123, 2001.
Hoffman, J. I. E. Incidence, mortality, and natural history. In: Pediatric Cardiology, edited by R. A. Anderson et al. London: Churchill Livingstone. 2002, pp. 111–139
Johnson, P., D. Maxwell, M. Tynan, and L. Allan. Intracardiac pressures in the human fetus. Heart 84:59–63, 2000.
Jonker, S., et al. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J. Appl. Physiol. 102:1130–1142, 2007.
Jouk, P., Y. Usson, G. Michalowicz, and L. Grossi. Three-dimensional cartography of the pattern of the myofibers in the second trimester fetal human heart. Anat. Embryol. 202:103–118, 2000.
Lin, D., and F. C. P. Yin. A multiaxial constitutive law for mammalian left ventricular myocardium in steady-state barium contracture or tetanus. ASME J. Biomech. Eng. 120:504–517, 1998.
McCartney, F. J., et al. (eds.). Pediatric Cardiology, 2nd ed. London: Churchill Livingstone, pp. 111–139, 2002.
McLean, M., and J. Prothero. Myofiber orientation in the weanling mouse heart. Am. J. Anat. 192:425–441, 1991.
McLean, M., M. A. Ross, and J. Prothero. Three dimensional reconstruction of the myofiber pattern in the fetal and neonatal mouse heart. Anat. Rec. 224:392–406, 1989.
Meyer-Wittkopf, M., A. Cole, S. G. Cooper, S. Schmidt, and G. H. Sholler. Three-dimensional quantitative echocardiographic assessment of ventricular volume in healthy human fetuses and in fetuses with congenital heart disease. J. Ultrasound Med. 20:317–327, 2001.
Molina, F. S., C. Faro, A. Sotiriadis, T. Dagklis, and H. Nicolaides. Heart stroke volume and cardiac output by four-dimensional ultrasound in normal fetuses. Ultrasound Obstet. Gynecol. 32:181–187, 2008.
Moulton, M. J., L. L. Creswell, S. W. Downing, R. L. Actis, B. A. Szabo, and M. K. Pasque. Myocardial material property determination in the in vivo heart using magnetic resonance imaging. Int. J. Card Imaging 12:153–167, 1996.
Ohayon, J., H. Cai, P. Jouk, Y. Usson, and A. Azancot. A model of structural and functional development of the normal human fetal left ventricle based on a global growth law. Comp. Meth. Biomech. Biomed. Eng. 2:113–126, 2002.
Ohayon, J., and R. Chadwick. Effects of collagen microstructure on the mechanics of the left ventricle. Biophys. J. 54:1077–1088, 1988.
Ohayon, J., Y. Usson, P. Jouk, and H. Cai. Fibre orientation in human fetal heart and ventricular mechanics: A small perturbation analysis. Comp. Meth. Biomech. Biomed. Eng. 2:83–105, 1999.
Okamoto, R. J., M. J. Moulton, S. J. Peterson, D. Li, and M. K. Pasque. Epicardial suction: a new approach to mechanical testing of the passive ventricular wall. ASME J. Biomech. Eng. 122:479–487, 2000.
Omens, J. H., K. D. May, and A. D. Mcculloch. Transmural distribution of three-dimensional strain in the isolated arrested canine left ventricle. Am. J. Physiol. (Heart Circ. Physiol.) 30:H918–H928, 1991.
Peña, E., M. A. Martínez, B. Calvo, and M. Doblaré. On the numerical treatment of initial strains in soft biological tissues. Int. J. Numer. Meth. Eng. 68:836–860, 2006.
Rychik, J. Fetal cardiovascular physiology. Pediatr. Cardiol. 25:201–209, 2004.
Siedner, S., M. Krüger, M. Schroeter, D. Metzler, W. Roell, B. K. Fleischmann, J. Hescheler, G. Pfitzer, and R. Stehle. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J. Physiol. 548:493–505, 2003.
Stalhand, J., A. Klarbring, and G. A. Holzapfel. Smooth muscle contraction: mechanochemical formulation for homogeneous finite strains. Progress Biophys. Mol. Biol. 96:465–481, 2008.
Stevens, C., E. Remme, I. LeGrice, and P. Hunter. Ventricular mechanics in diastole: material parameter sensitivity. J. Biomech. 36:737–748, 2003.
Taber, L. A. On a nonlinear theory for muscle shells: Part II. Application to the beating left ventricle. ASME J. Biomech. Eng. 113:63–71, 1991.
Taber, L. A., and S. Chabert. Theoretical and experimental study of growth and remodeling in the developing heart. Biomech. Model Mechanobiol. 1:29–43, 2002.
Tozeren, A. Static analysis of the left-ventricle. ASME J. Biomech. Eng. 105:39–46, 1983.
van Campen, D., J. Huyghe, P. Bovendeerd, and T. Arts. Biomechanics of the heart muscle. Eur. J. Mech. A 13:19–41, 1994.
Walker, J. C., M. B. Ratcliffe, P. Zhang, A. W. Wallance, B. Fata, E. W. Hsu, D. Saloner, and J. M. Guccione. MRI-based finite element analysis of left ventricular aneurysm. Am. J. Physiol. Heart Circ. Physiol. 289:H692–H700, 2005.
Weiwad, W. K., W. A. Linke, and M. H. Wussling. Sarcomere length–tension relationship of rat cardiac myocytes at lengths greater than optimum. J. Mol. Cell Cardiol. 32:247–259, 2000.
Acknowledgments
The authors thank Dr. Pierre-Simon Jouk (Department of Paediatric and Fetal Cardiology, Grenoble Hospital, France) and Y. Usson (TIMC Laboratory, Grenoble, France) for their useful discussions. Grants: The authors gratefully acknowledge research support from the European Community through the Sixth Framework Program through the DISHEART project FP6-2002-SME-1-513226 and the Spanish Ministry of Science and Technology through research projects DPI2007-63254, DPI2007-65601-C03-00, and SINBAD PSE-010000-2008, and the Instituto de Salud Carlos III (ISCIII) through the CIBER initiative. We thank also the Europe Program of Grants developed by Caja de Ahorros de la Inmaculada (CAI) and Diputación General de Aragón for their financial support to E. Peña.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Peña, E., Tracqui, P., Azancot, A. et al. Unraveling Changes in Myocardial Contractility During Human Fetal Growth: A Finite Element Analysis Based on In Vivo Ultrasound Measurements. Ann Biomed Eng 38, 2702–2715 (2010). https://doi.org/10.1007/s10439-010-0010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0010-x