Abstract
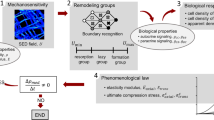
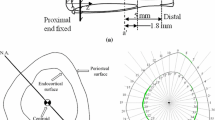
The process of external bone adaptation in cortical bone is modeled mathematically using finite element (FE) stress analysis coupled with an evolution model, in which adaptation response is triggered by mechanical stimulus represented by strain energy density. The model is applied to experiments in which a rat ulna is subjected to cyclic loading, and the results demonstrate the ability of the model to predict the bone adaptation response. The FE mesh is generated from micro-computed tomography (μCT) images of the rat ulna, and the stress analysis is carried out using boundary and loading conditions on the rat ulna obtained from the experiments [Robling, A. G., F. M. Hinant, D. B. Burr, and C. H. Turner. J. Bone Miner. Res. 17:1545–1554, 2002]. The external adaptation process is implemented in the model by moving the surface nodes of the FE mesh based on an evolution law characterized by two parameters: one that captures the rate of the adaptation process (referred to as gain); and the other characterizing the threshold value of the mechanical stimulus required for adaptation (referred to as threshold-sensitivity). A parametric study is carried out to evaluate the effect of these two parameters on the adaptation response. We show, following comparison of results from the simulations to the experimental observations of Robling et al. (J. Bone Miner. Res. 17:1545–1554, 2002), that splitting the loading cycles into different number of bouts affects the threshold-sensitivity but not the rate of adaptation. We also show that the threshold-sensitivity parameter can quantify the mechanosensitivity of the osteocytes.










Similar content being viewed by others
References
ABAQUS Inc., ABAQUS Version 6.7 Documentation. 2008.
Akhter, M. P., D. M. Raab, C. H. Turner, D. B. Kimmel, and R. R. Recker. Characterization of in vivo strain in the rat tibia during external application of a four-point bending load. J. Biomech. 25:1241–1246, 1992.
Altair Hypermesh. Altair HyperMesh Documentation. 2007.
Amira. Amira User’s Guide. 2005.
Beaupré, G. S., T. E. Orr, and D. R. Carter. An approach for time-dependent bone modeling and remodeling—theoretical development. J. Orthop. Res. 8:651–661, 1990.
Brebbia, C. A., and J. Dominguez. Boundary Elements: An Introductory Course. Southampton: WIT Press, 1989.
Burr, D. B., A. G. Robling, and C. H. Turner. Effects of biomechanical stress on bones in animals. Bone 30:781–786, 2002.
Carter, D. R., T. E. Orr, and D. P. Fyhrie. Relationships between loading history and femoral cancellous bone architecture. J. Biomech. 22:231–244, 1989.
Carter, D. R., M. C. H. Van der Meulen, and G. S. Beaupré. Mechanical factors in bone growth and development. Bone 18:S5–S10, 1996.
Cowin, S. C. (ed.). Bone Mechanics Handbook. Boca Raton: CRC Press, 2001.
Cowin, S. C., and K. Firoozbakhsh. Bone remodeling of diaphysial surfaces under constant load: theoretical predictions. J. Biomech. 14:471–484, 1981.
Cowin, S. C., and D. H. Hegedus. Bone remodeling I: theory of adaptive elasticity. J. Elasticity 6:313–325, 1976.
Discher, D. E., P. Janmey, and Y. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143, 2005.
Doblaré, M., and J. M. García. Anisotropic bone remodelling model based on a continuum damage-repair theory. J. Biomech. 35:1–17, 2002.
Fridez, P., L. Rakotomanana, A. Terrier, and P. F. Leyvraz. Three dimensional model of external bone adaptation. Comput. Methods Biomech. Biomed. Eng. 2:189–196, 1998.
Frost, H. M. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff’s law: the bone modeling problem. Anat. Rec. 226:403–413, 1990.
Frost, H. M. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: the remodeling problem. Anat. Rec. 226:414–422, 1990.
Gross, T. S., S. Srinivasan, C. C. Liu, T. L. Clemens, and S. D. Bain. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J. Bone Miner. Res. 17:493–501, 2002.
Han, Y., S. C. Cowin, B. M. Schaffler, and S. Weinbaum. Mechanotransduction and strain amplification in osteocyte cell processes. PNAS 101:16689–16694, 2004.
Hsieh, Y. F., and C. H. Turner. Effects of loading frequency on mechanically induced bone formation. J. Bone Miner. Res. 16:918–924, 2001.
Huiskes, R., R. Ruimerman, G. H. van Lenthe, and J. D. Janssen. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704–706, 2000.
Huiskes, R., H. Weinans, H. J. Grootenboer, M. Dalstra, B. Fudala, and T. J. Slooff. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J. Biomech. 20:1135–1150, 1987.
Knothe Tate, M. L. “Whither flows the fluid in bone?” An osteocyte’s perspective. J. Biomech. 36:1409–1424, 2003.
Knothe Tate, M. L., P. Niederer, and U. Knothe. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117, 1998.
Lanyon, L. E., A. E. Goodship, C. J. Pye, and J. H. MacFie. Mechanically adaptive bone remodelling. J. Biomech. 15:141–154, 1982.
Lanyon, L. E., and C. T. Rubin. Static vs dynamic loads as an influence on bone remodelling. J. Biomech. 17:897–905, 1984.
Levenston, M. E., and D. R. Carter. An energy dissipation-based model for damage stimulated bone adaptation. J. Biomech. 31:579–586, 1998.
Martinez, G., J. M. Garcia Aznar, M. Doblare, and M. Cerrolaza. External bone remodeling through boundary elements and damage mechanics. Math. Comput. Simul. 73:183–199, 2006.
McNamara, B. P., P. J. Prendergast, and D. Taylor. Prediction of bone adaptation in the ulnar-osteotomized sheep’s forelimb using an anatomical finite element model. J. Biomed. Eng. 14:209–216, 1992.
Pavalko, F. M., N. X. Chen, C. H. Turner, D. B. Burr, S. Atkinson, Y. Hsieh, J. Qiu, and R. L. Duncan. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. Cell Physiol. 275:C1591–C1601, 1998.
Prendergast, P. J., and D. Taylor. Prediction of bone adaptation using damage accumulation. J. Biomech. 27:1067–1076, 1994.
Robling, A. G., D. B. Burr, and C. H. Turner. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J. Bone Miner. Res. 15:1596–1602, 2000.
Robling, A. G., D. B. Burr, and C. H. Turner. Recovery periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol. 204:3389–3399, 2001.
Robling, A. G., F. M. Hinant, D. B. Burr, and C. H. Turner. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 17:1545–1554, 2002.
Rubin, C., and L. Lanyon. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. Am. 66:397–402, 1984.
Rubin, C. T., and L. E. Lanyon. Regulation of bone mass by mechanical strain magnitude Calcif. Tissue Int. 37:411–417, 1985.
Srinivasan, S., D. A. Weimer, S. C. Agans, S. D. Bain, and T. S. Gross. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res. 17:1613–1620, 2002.
Steck, R., P. Niederer, and M. L. Knothe Tate. A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J. Theor. Biol. 220:249–259, 2003.
Turner, C. H. Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407, 1998.
Turner, C., M. Forwood, and M. Otter. Mechanotransduction in bone: do bone cells act as sensors of fluid flow? FASEB J. 8:875–878, 1994.
van der Meulen, M. C. H., G. S. Beaupré, and D. R. Carter. Mechanobiologic influences in long bone cross-sectional growth. Bone 14:635–642, 1993.
Warden, S. J., and C. H. Turner. Mechanotransduction in cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone 34:261–270, 2004.
Weinans, H., R. Huiskes, and H. J. Grootenboer. The behavior of adaptive bone-remodeling simulation models. J. Biomech. 25:1425–1441, 1992.
Weinbaum, S., S. C. Cowin, and Y. Zeng. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27:339–360, 1994.
Zienkiewicz, O. C., and R. L. Taylor. The Finite Element Method. Volume 1—Basic Formulations and Linear Problems. London: McGraw-Hill, 1989.
Acknowledgments
We would like to thank Prof. Daniel Tortorelli of Department of Mechanical Science and Engineering, UIUC, for his help and advice on the smoothing filter approach, and Khanh Nguyen of Biomedical Engineering, Purdue, for the strain gage measurements. The support of the University of Illinois and the NIH through Grant AR046530 is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chennimalai Kumar, N., Dantzig, J.A., Jasiuk, I.M. et al. Numerical Modeling of Long Bone Adaptation due to Mechanical Loading: Correlation with Experiments. Ann Biomed Eng 38, 594–604 (2010). https://doi.org/10.1007/s10439-009-9861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9861-4