Abstract
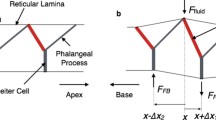
We present the results of a modeling study on the impact of mechanosensitive channels in the lateral wall of the outer hair cell on the cell frequency response. The model includes the electrical properties of the cell membrane, piezoelectricity associated with a membrane motor mechanism, and mechanosensitive channels in the cell lateral wall. The outer hair cell is loaded by the vibrating basilar and tectorial membranes, and this loading generates strain in the lateral wall. Our analysis reveals a property, the strain rate sensitivity, that, in concert with the piezoelectric effect, can enhance the cell frequency response. We discuss possible viscoelastic-type mechanisms of the channel’s strain rate sensitivity that is consistent with the organization of the composite cell lateral wall. The parameters of our model are chosen on the basis of the previously estimated electrical and piezoelectric properties as well as typical conductance and density of the mechanosensitive channels in cells. We found that the strain rate sensitivity of the channels can result in receptor potentials greater than those predicted by the RC (resistance and capacitance) analysis. The effect of the channels is especially significant in an intermediate range of sound frequencies, and the channel-related gain is up to 3–4 times between 3 and 15 kHz.
Similar content being viewed by others
References
Bett, G. C. L., and F. Sachs. Activation and inactivation of mechanosensitive channels in the chick heart. J. Membr. Biol. 173:237–254, 2000.
Brownell, W. E., C. R. Bader, D. Bertrand, and Y. de Ribaupierre. Evoked mechanical responses of isolated cochlear outer hair cell. Science 227:194–196, 1985.
Brownell, W. E., A. A. Spector, R. M. Raphael, and A. S. Popel. Micro- and nanomechanics of the cochlear outer hair cell. Annu. Rev. Biomed. Eng. 3:169–194, 2001.
Choe, Y., M. O. Magnasco, and A. J. Hudspeth. A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channels. Proc Natl Acad Sci USA 95:15321–15326, 1998.
Corey D. P., and A. J. Hudspeth. Response latency of vertebrate hair cells. Biophys. J. 26:499–506, 1979.
Dallos, P. Overview: Cochlear neurobiology. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer-Verlag, 1996, pp. 1–43.
Dallos, P., and B. N. Evans. High-frequency motility of outer hair cells and the cochlear amplifier. Science 267:2006–2009, 1995.
Ding, J. P., R. P. Salvi, and F. Sachs. Stretch-activated channels in guinea pig outer hair cell. Hear. Res. 56:19–28, 1991.
Evans, E., and R. Skalak. Mechanics and Thermodynamics of Biomembranes. Boca Raton: CRC Press, 1980, 254 pp.
Fabry, B., G. N. Maksym, J. P. Butler, M. Glogauer, D. Navajas, and J. J. Fredberg. Scaling the microreology of living cells. Phys. Rev. Lett. 87:148102 (1–4), 2001.
Frank, G., W. Hemmer, and A. W. Gummer. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. USA. 96:4420–4425, 1999.
Geisler, C. D. From Sound to Synapse. New York: Oxford University Press, 1998, 381 pp.
Geleoc, G. S.G, G. W. T. Lennan, G. P. Richardson, and C. R. Kros. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. R. Soc. Lond. B 264:611–621, 1997.
Hamill, O. P., and B. Martinac. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–739, 2001.
Housley, G. D. and J. F. Ashmore. Ionic currents of outer hair cells isolated from guinea-pig cochlea. J. Physiol. 448:73–98, 1992.
Iwasa, K. H., M. Li, M. Jia, and B. Kachar. Stretch sensitivity of the lateral wall of the auditory outer hair cell from the guinea pig. Nuerosci. Lett. 133:171–174, 1991.
Kros, C. J., A. Rusch, and G. P. Richardson. Mechanoelectrical transducer currents in hair cells of cultured neonatal mouse cochlea. Proc. R. Soc. Lond. B 249:185–193, 1992.
Kros, C. J. Physiology of mammalian cochlear hair cells. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer-Verlag, 1996, pp. 318–385.
Liberman, M. C., J. Gao, D. Z.-Z. He, X. Wu, S. Jia, and J. Zuo. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419:300–304, 2002.
Mammano, F., and J. F. Ashmore. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature 365:838–841, 1993
Mammano, F., and J. F. Ashmore. Differential expression of outer hair cell potassium currents in the isolated cochlea of the guinea-pig. J. Physiol. 496:639–646, 1996.
Markin, V. S., and A. J. Hudspeth. Gating-spring models of mechanoelectrical transduction by hair cells of the internal ear. Annu. Rev. Biophys. Biomol. Struct. 24:59–83, 1995.
McBride, D. W., and O. P. Hamill. Presure-clamp technique for measurement of the relaxation kinetics of mechanosensitive channels. Trends Neurosci. 16:341–345, 1993.
Morimoto, N., R. M. Raphael, A. Nygren, and W. E. Brownell. Excess plasma membrane and effects of ionic amphipaths on mechanics of outer hair cell lateral wall. Am. J. Physiol. Cell Physiol. 282:C1076–1086, 2002.
Morris, C. E. Mechanosensitive ion channels in eukaryotic cells. In: Cell Physiology. Source Book (second edition), edited by N. Sperelakis. San Diego: Academic Press, 1997, pp. 668–681.
Oliver, D., D. Z.-Z. He, N. Klocker, J. Ludwig, U. Schulte, S. Waldegger, J. P. Ruppersberg, P. Dallos, and B. Fakler. Intracellular anions as the voltage-sensor of Prestin, the outer hair cell motor protein. Science 292:2340–2343, 2001.
Ospeck, M., X.-X. Dong, and K. H. Iwasa. Limiting frequency of the cochlear amplifier based on electromotility of outer hair cell. Biophys. J. 84:739–749, 2003.
Ruggero, M. A. Responses to sound of the basilar membrane of the mammalian cochlea. Curr. Opin. Neurobiol. 2:449–456, 1992.
Russell, I. J., and K. E. Nilsen. The location of the cochlear amplifier: Spatial representationof a single tone of the guinea pig basilar membrane. Proc. Natl. Acad. Sci. USA. 94:2660–2664, 1997.
Rybalchenko, V., and J. Santos-Sacchi. Cl- flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J. Physiol. 547:873–891, 2003.
Sachs, F., and C. Morris. Mechanosensitive ion channels in non-specialized cells. In: Reviews of Physiology Biochemistry and Pharmacology, edited by M. P. Blaustein. Berlin: Springer-Verlag, 1998, pp. 1–78.
Santos-Sacchi, J. On the frequency limit and phase of outer hair cell motility: Effects of the membrane filter. J. Neurosci. 12:1906–1916, 1992.
Santos-Sacchi J, G. J. Huang, and M. Wu. Mapping the distribution of outer hair cell voltage-dependent conductances by electrical amputation. Biophys. J. 73:1424–1429, 1997.
Santos-Sacchi, J., S. Kakehata, T. Kikuchi, Y. Katory, and T. Takasaka. Density of motility-related charge in the outer hair cell of the guinea pig is inversely related to best frequency. Neurosci. Lett. 256:155–158, 1998.
Sato, M, D. P. Theret, L. T. Wheeler, N. Oshima, and R. M. Nerem. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J. Biomech. Eng. 112:263–268, 1990.
Sit, P. S., A. A. Spector, A. J. Lue, A. S. Popel, and W. E. Brownell. Micropipette aspiration on the outer hair cell lateral wall. Biophys. J. 72:2812–2819, 1997.
Spector, A. A., M. Ameen, R. A. Schmiedt. Modeling 3-D deformation of outer hair cells and their production of the active force in the cochlea. Biomech. Model. Mechanobiol. 1:123–135, 2002.
Spector, A. A., W. E. Brownell, and A. S. Popel. Effect of the membrane piezoelectric properties on the outer hair cell receptor potential under high-frequency conditions. In: Abstracts of the 29th Meeting of ARO, January, 2002, St. Petersburg Beach, Florida, 2002, p. 255.
Spector, A. A., W. E. Brownell, and A. S. Popel. Effect of outer hair cell piezoelectricity on high-frequency receptor potentials. J. Acoust. Soc. Am. 113:453–461, 2003.
Steele, C. R., G. Baker, J. A. Tolomeo, and D. Zetes. Electromechanical models of outer hair cell. In: Biophysics of Hair Cell Sensory Systems, edited by H. Duifhuis, J. W. Horst, P. van Dijk, and S. M. van Netten. Singapore: World Scientific Press, 1993, pp. 207–214.
Tolomeo, J. A., and C. R. Steele. Orthotropic piezoelectric properties of cochlear outer hair cell wall. J. Acoust. Soc. Am. 97:3006–3011, 1995.
Wang, N. Mechanical interaction among cytoskeletal filaments. Hypertension 32:162–165, 1998.
Wang, N., and D. E. Ingber. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 66:2181–2189, 1994.
Weitzel, E. K., R. Tasker, and W. E. Brownell. Outer hair cell piezoelectricity: Frequency response enhancement and resonance behavior. J. Acoust. Soc. Am. 114:1462–1466, 2003.
Zheng, J., W. Shen, D. Z.-Z. He, K. B. Long, L. D. Madison, and P. Dallos. Prestin is the motor protein of cochlear outer hair cell. Nature 405:149–155, 2000.
Zwislocki, J. J. Auditory Sound Transmission. Mahwah, NJ: Lawrence Erlbaum Associates, 2002, 419 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spector, A.A., Popel, A.S., Eatock, R.A. et al. Mechanosensitive Channels in the Lateral Wall Can Enhance the Cochlear Outer Hair Cell Frequency Response. Ann Biomed Eng 33, 991–1002 (2005). https://doi.org/10.1007/s10439-005-5749-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-5749-0