Abstract
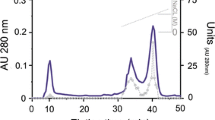
Three trypsin isoforms (designated as T1, T2, and T3), three chymotrypsin isoforms (Kh1, Kh2, and Kh3), and two elastase isoforms (E1 and E2) were isolated from the pancreas of European catfish Silurus glanis L. by salting out with (NH4)2SO4, gel chromatography on Sephadex G-75, and ion exchange chromatography on DEAE cellulose. Isoelectric points of the enzymes, determined by isoelectric focusing, amounted to 4.42 for T1, 5.64 for T2, 6.90 for T3, 4.93 for Kh1, 5.23 for Kh2, 6.18 for Kh3, 6.17 for E1, and 8.48 for E2. Molecular weights of proteinases within each group were close and amounted to 30,100 Da for trypsins, 39,800 Da for chymotrypsins, and 24,000 Da for elastases. The enzymes isolated displayed maximal activities at alkaline pH values. Inhibitor analysis demonstrated that all the proteinases isolated from European catfish pancreas belonged to the serine type.
Similar content being viewed by others
REFERENCES
Zhuravskaya, N.K. and Izotov, O.V., Myasnaya Industriya, 2002, no. 9, pp. 23–26.
Snitsar’, A., Chernukha, I., and Al’murziev, M., Mezhdunar. Agroprom. Zh., 1989, no. 6, pp. 118–121.
Glik, B. and Pasternak, Dzh., Molekulyarnaya biotekhnologiya: printsipy i primenenie (Molecular Biotechnology: Foundations and Use), Moscow: Mir, 2002.
Belen’kii, N.G., Polonskaya, L.B., and Chamin, N.N., Novoe v proizvodstve fermentov i fermentativnykh preparatov iz zhivotnogo syr’ya (Advances in Production of Enzymes and Enzymatic Preparations from Animal Raw Materials), Moscow: TsINTI Pishcheprom, 1966.
Mosolov, V.V., Proteoliticheskie Fermenty (Proteolytic Enzymes), Moscow: Nauka, 1971.
Kleine, R., Biologische Rundschau, 1969, no. 7, pp. 159–169.
Yakovenko, E.P., Klinich. Farm. Ter., 1998, no. 1, pp. 17–20.
Berezov, T.T., Sorosovskii Obrazovatel’nyi Zh., 1996, no. 3, pp. 23–27.
Borovskii, E.V., Klinicheskaya endodontiya (Clinical Root Canal Treatment), Moscow: AO Stomatologiya, 2003.
Kuznetsova, T.A., Markova, E.A., and Gonchar, A.I., Vestn. Ros. Ass. Akush.-Ginekol., 1997, no. 1, pp. 81–83.
Yoshinaka, R., Sato, M., Sato, T., and Ikeda, S., Comp. Biochem. Physiol., 1984, vol. 78B, no.3, pp. 569–573.
Simpson, B.K. and Haard, N.F., Comp. Biochem. Physiol., 1985, vol. 80B, no.3, pp. 475–480.
Khablyuk, V.V. and Proskuryakov, M.T., Prikl. Biokhim. Mikrobiol., 1983, vol. 19, no.3, pp. 403–407.
Tuppy, H., Wiesbauer, U., and Wintersberger, E., Hoppe Seylers Z. Physiol. Chem., 1962, vol. 329, nos.3–6, pp. 278–288.
Erlanger, B.F., Edel, F., and Cooper, A.G., Arch. Biochem. Biophys., 1966, vol. 115, pp. 206–210.
Katagiri, K., Takeuchi, T., Taniguchi, K., and Sasaki, M., Anal. Biochem., 1978, vol. 86, pp. 159–165.
Northrop, J.H., Kunitz, M., and Herriot, R.M., Crystalline Enzyme, New York: Columbia Univ., 1948.
Grant, N.H. and Robbins, K.C., Arch. Biochem. Biophys., 1957, vol. 66, pp. 396–403.
Pyatnitskii, N.P. and Selyukova, M.N., Vopr. Pitan., 1960, no. 2, pp. 42–48.
Righetti, P., Isoelectric Focusing. Theory, Methodology and Applications, Amsterdam: Elsevier, 1983. Translated under the title Izoelektricheskoe fokusirovanie: teoriya, metody i primenenie, Moscow: Mir, 1986.
Reeck, G.R., Winter, W.R., and Neurath, H., Biochemistry, 1970, vol. 9, no.6, pp. 1399–1403.
Ardelt, W., Biochim. Biophys. Acta, 1974, vol. 341, pp. 318–326.
Allan, B.J., Zagen, N.I., and Keller, P.J., Arch. Biochem. Biophys., 1970, vol. 136, pp. 529–540.
Asgeirsson, B. and Bjarnason, B., Comp. Biochem. Physiol., 1991, vol. 99B, pp. 327–335.
Kitamikado, M. and Tachino, S., Bull. Jpn. Soc. Sci. Fish., 1960, vol. 26, pp. 685–690.
Munilla-Moran, R. and Saborido-Rey, F., Comp. Biochem. Physiol., 1996, vol. 113B, pp. 818–826.
Yoshinaka, R., Sato, M., Suzuki, T., and Ikeda, S., Comp. Biochem. Physiol., 1984, vol. 77B, no.1, pp. 1–6.
Walsh, K.A., Metods Enzymol., New York: Academic, 1970, vol. 19, pp. 41–63.
Wilcox, P.E., Metods Enzymol., New York: Academic, 1970, vol. 19, pp. 64–108.
Smillie, L., Furka, A., Nagabhushan, N., and Stevenson, K., Nature, 1968, vol. 218, no.5139, pp. 343–346.
Colomb, E., Guy, O., Deprex, P., Michel, P., and Figarella, C., Biochim. Biophys. Acta, 1979, vol. 525, no.1, pp. 186–193.
Author information
Authors and Affiliations
Additional information
Translated from Prikladnaya Biokhimiya i Mikrobiologiya, Vol. 41, No. 2, 2005, pp. 158–164.
Original Russian Text Copyright © 2005 by Ulitina, Khablyuk, Proskuryakov.
Rights and permissions
About this article
Cite this article
Ulitina, N.N., Khablyuk, V.V. & Proskuryakov, M.T. Purification and properties of serine proteinases from European catfish Silurus glanis L. pancreas. Appl Biochem Microbiol 41, 139–144 (2005). https://doi.org/10.1007/s10438-005-0023-7
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10438-005-0023-7