Abstract
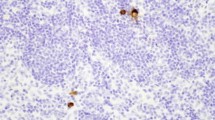
Background:The clinicopathologic significance of micrometastasis (MM) and tumor cell microinvolvement (TCM) in regional lymph nodes as identified by immunohistochemical staining for cytokeratin expression was evaluated in patients with node-negative gastric cancer.
Methods:MM was defined as tumor cells with stromal reaction, and TCM was defined as individual tumor cells without stromal reaction. We investigated 1761 lymph nodes obtained from 67 gastric cancer patients whose diagnosis showed no lymph node metastasis by routine histological examination. The depth of tumor invasion was T1 (submucosa) in 33 patients and T2 (muscularis propria and subserosa) in 34 patients. The lymph nodes were examined immunohistochemically for the presence of tumor cells using anti-cytokeratin AE1/AE3 monoclonal antibody. Both the biopsy tumor specimens obtained prior to surgery and the resected primary tumors were immunostained with E-cadherin (E-cad) monoclonal antibody.
Results:Thirty (1.5%) of the 1761 lymph nodes showed MM and/or TCM. MM with or without TCM was found in 10 patients, and TCM alone was found in 4 patients; 6 (18.2%) of the 33 patients with T1 tumor and 8 (23.5%) of the 34 patients with T2 tumor had occult lymph node metastasis. The 5-year survival rate was worse among those with MM with or without TCM, than among those without MM. Nearly all of the patients with MM and/or TCM had reduced or negative E-cad expression in the primary tumor.
Conclusions:We demonstrated that the incidence of MM and/or TCM in the lymph nodes of patients with gastric cancer is quite high, and that such metastasis is associated with the prognosis of patients with pN0. Examination of E-cad expression in biopsy tumor specimens may be useful for predicting MM and/or TCM.
Similar content being viewed by others
REFERENCES
Chen ZL, Perez S, Holmes EC, Wang HJ, Coulson WF, Wen DR, Cochran AJ. Frequency and distribution of occult micrometastases in lymph nodes of patients with non–small-cell lung carcinoma. J Natl Cancer Inst 1993; 85: 493–8.
Rasslick VV, Izbicki JR, Kubbuschok B, et al. Immunohistochemical assessment of individual tumor cells in lymph nodes with non–small-cell lung cancer. J Clin Oncol 1994; 12: 1827–32.
Nasser IA, Lee AK, Bosari S, Saganich R, Heatley G, Silverman ML. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol 1993; 24: 950–7.
McGuckin MA, Cummings MC, Walsh MD, Hohn BG, Bennett IC, Wright RG. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer 1996; 73: 88–95.
Yokoyama N, Shirai Y, Hatakeyama K. Immunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibody. Cancer 1999; 85: 1465–9.
Natsugoe S, Mueller J, Stein HJ, Feith M, Hofler H, Siewert JR. Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer 1998; 83: 858–66.
Maehara Y, Oshiro T, Endo K, et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery 1996; 119: 397–402.
Ishida K, Katsuyama T, Sugiyama A, Kawasaki S. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer 1997; 79: 1069–76.
Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, MartinEWJr. Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer 1994; 73: 563–9.
Jeffers MD, O’Dowd GM, Mulchahy H, Stagg M, O’Donoghue DP, Toner M. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol 1994; 172: 183–7.
Sasaki M, Watanabe H, Jass JR, et al. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in “node-negative” colorectal cancer. J Gastroenterol 1997; 32: 758–64.
Mayer B, Johnson JP, Leitl F, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res 1993; 53: 1690–5.
Kinsella AR, Green B, Lepts GC, Hill CL, Bowie G, Taylor BA. The role of the cell-cell adhesion molecule E-cadherin in large bowel tumor cell invasion and metastasis. Br J Cancer 1993; 67: 904–9.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer 1998; 1: 1–15.
Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline Phosphatase and monoclonal anti-alkaline Phosphatase (APAAP complexes). J Histochem Cytochem 1984; 32: 219–29.
Natsugoe S, Nakashima S, Matsumoto M, et al. Paraaortic lymph node micrometastasis and tumor cell microinvolvement in advanced gastric carcinoma. Gastric Cancer 1999; 2: 179–85.
Shiozaki H, Tahata H, Oka H, et al. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol 1991; 139: 17–23.
Oka H, Shiozaki H, Kobayashi K, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 1993; 53: 1696–1701.
Sievert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg 1996; 83: 1144–7.
Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000; 88: 577–83.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakajo, A., Natsugoe, S., Ishigami, S. et al. Detection and Prediction of Micrometastasis in the Lymph Nodes of Patients With pN0 Gastric Cancer. Ann Surg Oncol 8, 158–162 (2001). https://doi.org/10.1007/s10434-001-0158-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-001-0158-6