Abstract
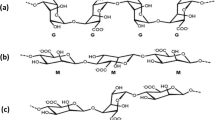
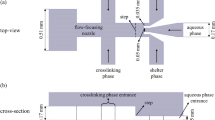
Alginate-based hydrogels are frequently employed in biomedical fields, where their size and uniformity are important for applications such as encapsulating pharmaceuticals or biological agents. In this paper, we study alginate droplet formation in a flow-focusing microfluidic device with a rectangular orifice of \(30\times 100\) microns in the presence of different types of surfactants in the continuous phase. This study looks at how surfactant type and concentration affect the generation of alginate microdroplets using droplet-based microfluidics, as well as how they affect the created alginate gel beads. To make alginate hydrogels, we used an external ionic cross-linking approach. Alginate hydrogels are formed by on-chip emulsifying a sodium alginate solution in an oil phase and collecting the emulsions in a bath containing calcium ions. Off-chip introduction of the cross-linking agents will reduce clogging issues that are common in droplet microfluidics. The diameter of microdroplets varies depending on the surfactant added to the continuous phase, either Span 80 or Tween 80, and the flow rate ratio between the dispersed and continuous phases. The conditions under which microdroplets form (the flow rate of the continuous phase and the surfactant supplied to it) determine the degree of gelation, shape, size, and monodipersity of hydrogels. Tween 80 emulsification results in the most homogeneous, totally gelled, tail-shaped hydrogels, whereas Span 80 emulsification results in smaller hydrogels with a more irregular size distribution. Using Span 80 resulted in partial gelation, necessitating a change in the collection bath (such as adding ethyl acetate to the oil phase).










Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Capretto L, Mazzitelli S, Balestra C, Tosi A, Nastruzzi C (2008) Effect of the gelation process on the production of alginate microbeads by microfluidic chip technology. Lab Chip 8(4):617–621
Chaemsawang W, Prasongchean W, Papadopoulos KI, Sukrong S, Kao WJ, Wattanaarsakit P (2018) Emulsion cross-linking technique for human fibroblast encapsulation. International Journal of Biomaterials 2018:
Chen M, Bolognesi G, Vladisavljević GT (2021) Crosslinking strategies for the microfluidic production of microgels. Molecules 26(12):3752
Cubaud T, Mason TG (2008) Capillary threads and viscous droplets in square microchannels. Phys Fluids 20(5):053302
Damiati S (2020) In situ microfluidic preparation and solidification of alginate microgels. Macromol Res 28(11):1046–1053
Davarcı F, Turan D, Ozcelik B, Poncelet D (2016) The influence of solution viscosities and surface tension on calcium-alginate microbead formation using dripping technique. Food Hydrocolloids 62:
Fu T, Wu Y, Ma Y, Li HZ (2012) Droplet formation and breakup dynamics in microfluidic flow-focusing devices: From dripping to jetting. Chem Eng Sci 84:207–217
He T, Wang W, Chen B, Wang J, Liang Q, Chen B (2020) 5-fluorouracil monodispersed chitosan microspheres: Microfluidic chip fabrication with crosslinking, characterization, drug release and anticancer activity. Carbohydrate polymers 236:116094
Hu Y, Azadi G, Ardekani AM (2015) Microfluidic fabrication of shape-tunable alginate microgels: Effect of size and impact velocity. Carbohydrate Polym 120:38–45
Hu Y, Azadi G, Ardekani AM (2015) Microfluidic fabrication of shape-tunable alginate microgels: Effect of size and impact velocity. Carbohydrate polymers 120:38–45
Hu Y, Wang Q, Wang J, Zhu J, Yang Y (2012) Shape controllable microgel particles prepared by microfluidic combining external ionic crosslinking. Biomicro Fluid 6:26502–265029
Huang K-S, Lai T-H, Lin Y-C (2007) Using a microfluidic chip and internal gelation reaction for monodisperse calcium alginate microparticles generation. Front Biosci-Landmark 12(8):3061–3067
Jiang B, Guo H, Chen D, Zhou M (2022) Microscale investigation on the wettability and bonding mechanism of oxygen plasma-treated pdms microfluidic chip. Appl Surface Sci 574:151704
Kaygusuz H, Evingür GA, Pekcan Ö, von Klitzing R, Erim FB (2016) Surfactant and metal ion effects on the mechanical properties of alginate hydrogels. Int J Biol Macromol 92:220–224
Kim JH, Jeon TY, Choi TM, Shim TS, Kim S-H, Yang S-M (2014) Droplet microfluidics for producing functional microparticles. Langmuir 30(6):1473–1488
Kim C, Park K-S, Kim J, Jeong S-G, Lee C-S (2017) Microfluidic synthesis of monodisperse pectin hydrogel microspheres based on in situ gelation and settling collection. J Chem Technol Biotechnol 92(1):201–209
Kiratzis I, Kovalchuk NM, Simmons MJ, Vigolo D (2022) Effect of surfactant addition and viscosity of the continuous phase on flow fields and kinetics of drop formation in a flow-focusing microfluidic device. Chem Eng Sci 248:117183
Lin Y-S, Yang C-H, Hsu Y-Y, Hsieh C-L (2013) Microfluidic synthesis of tail-shaped alginate microparticles using slow sedimentation. Electrophoresis 34(3):425–431
Liu Y, Tottori N, Nisisako T (2018) Microfluidic synthesis of highly spherical calcium alginate hydrogels based on external gelation using an emulsion reactant. Sensors and Actuators B: Chemical 283:
Lupo B, Maestro A, Porras M, Gutiérrez JM, González C (2014) Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food HydrocolL 38:56–65
Ma T, Gao X, Dong H, He H, Cao X (2017) High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or diels-alder click chemistry for cell encapsulation and delivery. Appl Mater Today 9:49–59
Marquis M, Davy J, Fang A, Renard D (2014) Microfluidics-assisted diffusion self-assembly: Toward the control of the shape and size of pectin hydrogel microparticles. Biomacromole 15(5):1568–1578
National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 8857, Ethyl acetate. https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-acetate/ (accessed March.14, 2022)
Paiboon N, Surassmo S, Ruktanonchai UR, Soottitantawat A (2022) Hydrodynamic control of droplet formation in narrowing jet and tip streaming regime using microfluidic flow-focusing. Int J Multiphase Flow 150:104013
Peng L, Yang M, Guo S-S, Liu W, X-z Zhao (2011) The effect of interfacial tension on droplet formation in flow-focusing microfluidic device. Biomed Microdev 13(3):559–564
Ratledge C, Dawson PSS, Rattray J (1984) Biotechnol Oils Fats Indus, vol 11. The American Oil Chemists Society, USA
Sadeghi D, Solouk A, Samadikuchaksaraei A, Seifalian AM (2021) Preparation of internally-crosslinked alginate microspheres: Optimization of process parameters and study of ph-responsive behaviors. Carbohydrate Polymers 255:117336
Sinzato YZ, Dias NJS, Cunha FR (2017) An experimental investigation of the interfacial tension between liquid-liquid mixtures in the presence of surfactants. Exp Thermal Fluid Sci 85:370–378
Vicini S, Mauri M, Wichert J, Castellano M (2017) Alginate gelling process: Use of bivalent ions rich microspheres. Polymer Eng Sci 57(6):531–536
Voo W-P, Ooi C-W, Islam A, Tey B-T, Chan E-S (2016) Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Europe Polymer J 75:343–353
Wang Q, Liu S, Yang F, Gan L, Yang X, Yang Y (2017) Magnetic alginate microspheres detected by mri fabricated using microfluidic technique and release behavior of encapsulated dual drugs. Int J Nanomed 12:4335
Wang Q, Zhang D, Xu H, Yang X, Shen A, Yang Y (2012) Microfluidic one-step fabrication of radiopaque alginate microgels with in situ synthesized barium sulfate nanoparticles. Lab Chip 12:4781–6
Yeh C-H, Zhao Q, Lee S-J, Lin Y-C (2009) Using a t-junction microfluidic chip for monodisperse calcium alginate microparticles and encapsulation of nanoparticles. Sens Actuat Phys 151(2):231–236
Yu D, Dong Z, Lim H, Chen Y, Ding Z, Sultana N, Wu J, Qin B, Cheng J, Li W (2019) Microfluidic preparation, shrinkage, and surface modification of monodispersed alginate microbeads for 3d cell culture. RSC Adv 9(20):11101–11110
Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC, Kumacheva E (2006) Microfluidic production of biopolymer microcapsules with controlled morphology. Journal of the american chemical societyJ Am Chem Soc 128(37):12205–12210
Zou Q, Hou F, Wang H, Liao Y, Wang Q, Yang Y (2019) Microfluidic one-step preparation of alginate microspheres encapsulated with in situ-formed bismuth sulfide nanoparticles and their photothermal effect. Europe Polym J 115:282–289
Acknowledgements
This work was supported by the UK Research and Innovation, Research England, International investment initiative (I3) [3DMED] and by INSF [98001236]. We would like to thank Prof. Matteo Santin, and Prof. Alidad Amirfazli for helpful discussions.
Funding
Partial financial support was received from UKRI (UK Research and Innovation).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows. Conceptualization : M. Oveysi, G. Peregrino , Dr. V. Bazargan, Prof. M. Marengo. Investigation : M. Oveysi:, A, Zaker, G. Peregrino. Software: A. Zaker, G. Peregrino, Dr. V. Bazargan. Visualization: M. Oveysi, A. Zaker. Writing - original draft: M. Oveysi. Writing - review & editing: A. Zaker, G. Peregrino , Dr. V. Bazargan, Prof. M. Marengo. Funding acquisition: Prof. M. Marengo.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (avi 1679 KB)
Supplementary file 2 (avi 4116 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oveysi, M., Zaker, M.A., Peregrino, G. et al. Droplet-based fabrication of alginate hydrogel microparticles in presence of surfactants. Microfluid Nanofluid 27, 45 (2023). https://doi.org/10.1007/s10404-023-02655-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-023-02655-2