Abstract
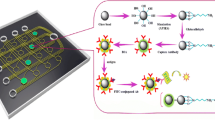
A simple valveless microfluidic device consisting of a reaction chamber and eight flow channels was fabricated for the detection of proteins. The efficient exchange of solutions in the reaction chamber was achieved by sequentially introducing solutions into the reaction chamber and removing them from the reaction chamber through the same flow channels. The influence of the hydrophobicity of the reaction chamber and the viscosity of a blocking solution on solution exchange was investigated. The analytical performance of the device was examined by detecting human interleukin 2 (IL-2) using a sandwich fluorescence immunoassay, with the amount of immuno-complex formed in the reaction chamber monitored by fluorescence microscopy. The approach facilitated the rapid detection of IL-2, in which the total assay time was less than 30 min. A clear dependence of fluorescence intensity on IL-2 concentration was observed over the 125 pg/mL–2.0 ng/mL range, with a detection limit of 105 pg/mL. To promote automation, a simple mechanism that exchanges solutions by the push–pull movement of a PDMS diaphragm was also demonstrated.







Similar content being viewed by others
References
Acres RG, Ellis AV, Alvino J, Lenahan CE, Khodakov DA, Metha GF, Andersson GG (2012) Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J Phys Chem C 116:6289–6297
Bange A, Halsall HB, Heineman WR (2005) Microfluidic immunosensor systems. Biosens Bioelectron 20:2488–2503
Barbosa AI, Reis NM (2017) A critical insight into the development pipeline of microfluidic immunoassay devices for the sensitive quantitation of protein biomarkers at the point of care. Analyst 142:858–882
Benard WL, Kahn H, Heuer AH, Huff MA (1998) Thin-film shape-memory alloy actuated micropumps. J Microelectromech Syst 7:245–251
Berktas M, Guducuoglu H, Bozkurt H, Onbasi K, Kurtoglu M, Andic S (2004) Change in serum concentrations of interleukin-2 and interferon-γ during treatment of tuberculosis. J Int Med Res 32:324–330
Bratten CDT, Cobbold PH, Cooper JM (1997) Micromachining sensors for electrochemical measurement in subnanoliter volumes. Anal Chem 69:253–258
Findlay JWA et al (2000) Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal 21:1249–1273
Giri B, Pandey B, Neupane B, Ligler FS (2016) Signal amplification strategies for microfluidic immunoassays. Trac Trends Anal Chem 79:326–334
Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE (2012) Point of care diagnostics: status and future. Anal Chem 84:487–515
Han SW, Koh W-G (2016) Hydrogel-framed nanofiber matrix integrated with a microfluidic device for fluorescence detection of matrix metalloproteinases-9. Anal Chem 88:6247–6253
Hua B et al (2014) An improved surface passivation method for single-molecule studies. Nat Methods 11:1233–1236
Huckle D (2008) Point-of-care diagnostics: an advancing sector with nontechnical issues. Expert Rev Mol Diagn 8:679–688
Iverson BD, Garimella SV (2008) Recent advances in microscale pumping technologies: a review and evaluation. Microfluid Nanofluidics 5:145–174
Juncker D et al (2002) Autonomous microfluidic capillary system. Anal Chem 74:6139–6144
Jung W, Han J, Choi J-W, Ahn CH (2015) Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron Eng 132:46–57
Lai S, Wang S, Luo J, Lee LJ, Yang S-T, Madou MJ (2004) Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal Chem 76:1832–1837
Laxminarayan R et al (2006) Advancement of global health: key messages from the Disease Control Priorities Project. Lancet 367:1193–1208
Linder V, Sia SK, Whitesides GM (2005) Reagent-loaded cartridges for valveless and automated fluid delivery in microfluidic devices. Anal Chem 77:64–71
Liu Y, Wang H, Huang J, Yang J, Liu B, Yang P (2009) Microchip-based ELISA strategy for the detection of low-level disease biomarker in serum. Anal Chim Acta 650:77–82
Luppa PB, Müller C, Schlichtiger A, Schlebusch H (2011) Point-of-care testing (POCT): current techniques and future perspectives. Trends Anal Chem 30:887–898
Lynn NS, Henry CS, Dandy DS (2009) Evaporation from microreservoirs. Lab Chip 9:1780–1788. https://doi.org/10.1039/b900556k
Moerman R, Knoll J, Apetrei C, van den Doel LR, van Dedem GWK (2005) Quantitative analysis in nanoliter wells by prefilling of wells using electrospray deposition followed by sample introduction with a coverslip method. Anal Chem 77:225–231
Nashida N, Satoh W, Fukuda J, Suzuki H (2007) Electrochemical immunoassay on a microfluidic device with sequential injection and flushing functions. Biosens Bioelectron 22:3167–3173. https://doi.org/10.1016/j.bios.2007.02.010
Ng AHC, Uddayasankar U, Wheeler AR (2010) Immunoassays in microfluidic systems. Anal Bioanal Chem 397:991–1007
Novo P, Volpetti F, Chu V, Conde JP (2013) Control of sequential fluid delivery in a fully autonomous capillary microfluidic device. Lab Chip 13:641–645
Obata H, Kuji T, Kojima K, Sassa F, Yokokawa M, Takekosh K, Suzuki H (2016) Electrochemical bubble-based bidirectional microfluidic transport. ACS Sens 1:190–196. https://doi.org/10.1021/acssensors.5b00059
Pai NP, Vadnais C, Denkinger C, Engel N, Pai M (2012) Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 9:e1001306
Richter A, Klatt S, Paschew G, Klenke C (2009) Micropumps operated by swelling and shrinking of temperature-sensitive hydrogels. Lab Chip 9:613–618
Sassa F, Al-Zain Y, Ginoza T, Miyazaki S, Suzuki H (2012) Miniaturized shape memory alloy pumps for stepping microfluidic transport. Sens Actuators B 165:157–163
Shimizu Y, Takashima A, Satoh W, Sassa F, Fukuda J, Suzuki H (2009) Biochip with integrated pumps for plug-based sequential exchange of solutions. Sens Actuators B 140:649–655
Suzuki H, Yoneyama R (2003) Integrated microfluidic system with electrochemically actuated on-chip pumps and valves. Sens Actuators B 96:38–45. https://doi.org/10.1016/S0925-4005(03)00482-9
Suzuki H, Tokuda T, Kobayashi K (2002) A disposable “intelligent mosquito” with a reversible sampling mechanism using the volume-phase transition of a gel. Sens Actuators B 83:53–59
Usuba R et al (2016) Photonic Lab-on-a-Chip for rapid cytokine detection. ACS Sens 1:979–986
Vandenberg ET, Bertilsson L, Liedberg B, Uvdal K, Erlandsson R, Elwing H, Lundström I (1991) Structure of 3-aminopropyl triethoxy silane on silicon oxide. J Colloid Interface Sci 147:103–118
Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT (2015) Emerging technologies for next-generation point-of-care testing. Trends Biotechnol 33:692–705
Yu L, Li CM, Zhou Q, Luong JHT (2007) Poly(vinyl alcohol) functionalized poly(dimethylsiloxane) solid surface for immunoassay. Bioconjugate Chem 18:281–284
Yu ZTF et al (2015) Rapid, automated, parallel quantitative immunoassays using highly integrated microfluidics and AlphaLISA. Sci Rep 5:11339
Zimmermann M, Delamarche E, Wolf M, Hunziker P (2005) Modeling and optimization of high-sensitivity low-volume microfluidic-based surface immunoassays. Biomed Microdevices 7:99–110
Acknowledgements
We would like to thank Professor Masatoshi Yokokawa and Dr. Gokul Chandra Biswas of University of Tsukuba for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (WMV 688 KB)
Supplementary material 3 (WMV 1354 KB)
Rights and permissions
About this article
Cite this article
Pramanik, S.K., Suzuki, H. Microfluidic device with a push–pull sequential solution-exchange function for affinity sensing. Microfluid Nanofluid 23, 19 (2019). https://doi.org/10.1007/s10404-019-2188-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-019-2188-z