Abstract
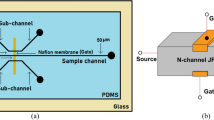
An ion concentration polarization-based microfluidic sample preconcentration chip consisting of a polydimethylsiloxane microchannel and a graphene oxide (GO)–Nafion nanomembrane is fabricated using conventional MEMS techniques. The performance of the proposed device is evaluated using a fluorescein sample with an initial concentration of 10−5 M for membranes with three different GO–Nafion volume ratios (2:1, 3:1 and 4:1) and four different GO concentrations (0.3, 0.5, 1 and 2 wt%). It is shown that for a GO concentration of 0.3 wt%, a maximum preconcentration factor of approximately 50-fold is achieved using a GO–Nafion volume ratio of 3:1. Moreover, for the same volume ratio (3:1), a 60-fold enhancement of the sample concentration is obtained given a GO concentration of 0.5 wt%. Overall, the results show that compared to a pure Nafion membrane, the addition of GO yields an effective improvement in the sample preconcentration factor (i.e., 60-fold vs. 40-fold), but at the expense of a longer preconcentration time (30 min vs. 6 min 36 s). The superior concentration performance of the GO–Nafion membrane is attributed to the effects of dissociated carboxylate ions in attracting a greater number of cations into the membrane nanopores.














Similar content being viewed by others
References
Anderson NL, Anderson NG (2002) The human plasma proteome history, character, and diagnostic prospects. Mol Cell Proteomics 1:845–867
Anderson JR, Chiu DT, Wu H, Schueller O, Whitesides GM (2000) Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 21:27–40
Astorga-Wells J, Swerdlow H (2003) Fluidic preconcentrator device for capillary electrophoresis of proteins. Anal Chem 75:5207–5212
Burgi DS, Chien RL (1991) Optimization in sample stacking for high-performance capillary electrophoresis. Anal Chem 63:2042–2047
Chao C-C, Chiu P-H, Yang R-J (2015) Preconcentration of diluted biochemical samples using microchannel with integrated nanoscale Nafion membrane. Biomed Microdevices 17:1–9
Chen Y-Y, Chiu P-H, Weng C-H, Yang R-J (2016) Preconcentration of diluted mixed-species samples following separation and collection in a micro-nanofluidic device. Biomicrofluidics 10:014119
Chiu P-H, Weng C-H, Yang R-J (2015) Preconcentration and separation of mixed-species sample near a nano-junction in a convergent microchannel. Sensors 15:30704–30715
Dhopeshwarkar R, Crooks RM, Hlushkou D, Tallarek U (2008) Transient effects on microchannel electrokinetic filtering with an ion-permselective membrane. Anal Chem 80:1039–1048
Feng K, Tang B, Wu P (2014) Sulfonated graphene oxide–silica for highly selective Nafion-based proton exchange membranes. J Mater Chem A 2:16083–16092
Gebauer P, Boček P (2002) Recent progress in capillary isotachophoresis. Electrophoresis 23:3858–3864
Hatch AV, Herr AE, Throckmorton DJ, Brennan JS, Singh AK (2006) Integrated preconcentration SDS-PAGE of proteins in microchips using photopatterned cross-linked polyacrylamide gels. Anal Chem 78:4976–4984
Hlushkou D, Dhopeshwarkar R, Crooks RM, Tallarek U (2008) The influence of membrane ion-permselectivity on electrokinetic concentration enrichment in membrane-based preconcentration units. Lab Chip 8:1153–1162
Hoeman KW, Lange JJ, Roman GT, Higgins DA, Culbertson CT (2009) Electrokinetic trapping using titania nanoporous membranes fabricated using sol–gel chemistry on microfluidic devices. Electrophoresis 30:3160–3167
Khandurina J, Jacobson SC, Waters LC, Foote RS, Ramsey JM (1999) Microfabricated porous membrane structure for sample concentration and electrophoretic analysis. Anal Chem 71:1815–1819
Kim SJ, Wang Y-C, Lee JH, Jang H, Han J (2007) Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys Rev Lett 99:044501
Kim P, Kim SJ, Han J, Suh KY (2009a) Stabilization of ion concentration polarization using a heterogeneous nanoporous junction. Nano Lett 10:16–23
Kim SJ, Li LD, Han J (2009b) Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir 25:7759–7765
Kim SJ, Ko SH, Kang KH, Han J (2010a) Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 5:297–301
Kim SJ, Song Y-A, Han J (2010b) Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: theory, fabrication, and applications. Chem Soc Rev 39:912–922
Kumar R, Xu C, Scott K (2012) Graphite oxide/Nafion composite membranes for polymer electrolyte fuel cells. RSC Adv 2:8777–8782
Lee JH, Song Y-A, Han J (2008) Multiplexed proteomic sample preconcentration device using surface-patterned ion-selective membrane. Lab Chip 8:596–601
Manz A, Graber N, Há Widmer (1990) Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens Actuators B Chem 1:244–248
McDonald JC, Whitesides GM (2002) Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res 35:491–499
Oleschuk RD, Shultz-Lockyear LL, Ning Y, Harrison DJ (2000) Trapping of bead-based reagents within microfluidic systems: on-chip solid-phase extraction and electrochromatography. Anal Chem 72:585–590
Phan DT, Yang C, Nguyen NT (2015) Fabrication of nanoporous junctions using off-the-shelf Nafion membrane. J Micromech Microeng 25:115019
Phan DT, Shaegh SAM, Yang C, Nguyen NT (2016) Sample concentration in a microfluidic paper-based analytical device using ion concentration polarization. Sens Actuators B 222:735–740
Pu Q, Yun J, Temkin H, Liu S (2004) Ion-enrichment and ion-depletion effect of nanochannel structures. Nano Lett 4:1099–1103
Quirino JP, Terabe S (1998) Exceeding 5000-fold concentration of dilute analytes in micellar electrokinetic chromatography. Science 282:465–468
Schoch RB, Han J, Renaud P (2008) Transport phenomena in nanofluidics. Rev Mod Phys 80:839–883
Song S, Singh AK, Kirby BJ (2004) Electrophoretic concentration of proteins at laser-patterned nanoporous membranes in microchips. Anal Chem 76:4589–4592
Stroock AD, Whitesides GM (2002) Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis 23:3461–3473
Wang Y-C, Han J (2008) Pre-binding dynamic range and sensitivity enhancement for immuno-sensors using nanofluidic preconcentrator. Lab Chip 8:392–394
Wang Y, Pant K, Chen Z, Wang G, Diffey WF, Ashley P, Sundaram S (2009) Numerical analysis of electrokinetic transport in micro-nanofluidic interconnect preconcentrator in hydrodynamic flow. Microfluid Nanofluid 7:683–696
Yu C, Davey MH, Svec F, Fréchet JM (2001) Monolithic porous polymer for on-chip solid-phase extraction and preconcentration prepared by photoinitiated in situ polymerization within a microfluidic device. Anal Chem 73:5088–5096
Yu H, Lu Y, Y-g Zhou, F-b Wang, F-y He, X-h Xia (2008) A simple, disposable microfluidic device for rapid protein concentration and purification via direct-printing. Lab Chip 8:1496–1501
Acknowledgements
The authors gratefully acknowledge the financial support provided to this study by the Ministry of Science and Technology of Taiwan under Project Number MOST 103-2221-E-006-093-MY3. The acknowledgement is extended to the National Nano-Device Laboratory for providing microfabrication facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, CH., Yang, RJ. Enhanced sample preconcentration in microfluidic chip using graphene oxide–Nafion membrane. Microfluid Nanofluid 20, 168 (2016). https://doi.org/10.1007/s10404-016-1835-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-016-1835-x