Abstract
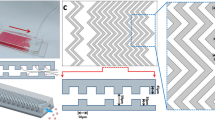
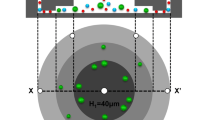
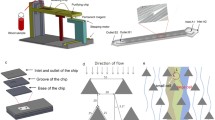
Isolation of the extremely rare circulating tumor cells (CTCs) from peripheral blood has become an effective tool for monitoring and staging tumor development, based on which the therapeutic efficacy can be evaluated. Many techniques have been developed for CTC isolation and enumeration. However, isolation and detection of CTCs with high sensitivity remains a challenging work, and there still exists an urgent demand to retrieve tumor cells after isolation for further molecular and cellular analyses. Herein, we report a dual-functional microchip with double parallel layers of herringbone structure. CTC isolation and retrieving can be simply achieved based on the reversible conjugation with anti-EpCAM modified on the interior channel surface. Specifically, two major strategies were engaged to improve the CTC isolation based on the synergistic effect of significantly increased functional surface area and local microvortex mixing. This approach could achieve CTC isolation efficiency of 75% on average and the detection limit down to 10 cells mL−1. Furthermore, CTC retrieving rate was found at 73.4%. Therefore, the present method has a good potential for quantitation and retrieving CTCs with high efficiency for precise cancer diagnosis and subsequent cellular and molecular analyses.






Similar content being viewed by others
References
Alazzam A, Stiharu I, Bhat R et al (2011) Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis 32:1327–1336
Alix-Panabieres C, Pantel K (2014) Challenges in circulating tumour cell research. Nat Rev Cancer 14:623–631
Andree KC, Barradas AMC, Nguyen AT et al (2016) Capture of tumor cells on anti-EpCAM-functionalized poly(acrylic acid)-coated surfaces. ACS Appl Mater Interfaces 8:14349–14356
Arya SK, Lim B, Rahman AR (2013) Enrichment, detection and clinical significance of circulating tumor cells. Lab Chip 13:1995–2027
Balic M, Williams A, Lin H et al (2013) Circulating tumor cells: from bench to bedside. Annu Rev Med 64:31–44
Barok M, Szollosi J (2011) Steps in metastasis research: analyzing, collecting, and culturing circulating tumor cells. Cytom Part A 79:93–94
Bernards R, Weinberg RA (2002) Metastasis genes: a progression puzzle. Nature 418:823
Caceres G, Puskas JA, Magliocco AM (2015) Circulating tumor cells: a window into tumor development and therapeutic effectiveness. Cancer Control 22:167–176
den Toonder J (2011) Circulating tumor cells: the Grand Challenge. Lab Chip 11:375–377
Deng Y, Zhang Y, Sun S et al (2014) An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Sci Rep UK 4:7499
Devriese LA, Bosma AJ, van de Heuvel MM et al (2012) Circulating tumor cell detection in advanced non-small cell lung cancer patients by multi-marker QPCR analysis. Lung Cancer 75:242–247
Friend J, Yeo L (2010) Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 4:026502
Gascoyne PR, Shim S (2014) Isolation of circulating tumor cells by dielectrophoresis. Cancers 6:545–579
Giuliano M, Giordano A, Jackson S et al (2014) Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res 16:440
Gleghorn JP, Pratt ED, Denning D et al (2010) Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip 10:27–29
Gorges TM, Pantel K (2013) Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother 62:931–939
Green BJ, Safaei TS, Mepham A et al (2016) Beyond the capture of circulating tumor cells: next-generation devices and materials. Angew Chem Int Edit 55:1252–1265
Hirsch JD, Eslamizar L, Filanoski BJ et al (2002) Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation. Anal Biochem 308:343–357
Hosokawa M, Arakaki A, Takahashi M et al (2009) High-density microcavity array for cell detection: single-cell analysis of hematopoietic stem cells in peripheral blood mononuclear cells. Anal Chem 81:5308–5313
Kang JH, Krause S, Tobin H et al (2012) A combined micromagnetic-microfluidic device for rapid capture and culture of rare circulating tumor cells. Lab Chip 12:2175–2181
Kim S, Han SI, Park MJ et al (2013) Circulating tumor cell microseparator based on lateral magnetophoresis and immunomagnetic nanobeads. Anal Chem 85:2779–2786
Kralj JG, Arya C, Tona A et al (2012) A simple packed bed device for antibody labelled rare cell capture from whole blood. Lab Chip 12:4972–4975
Li Y, Gong J, Zhang Q et al (2016) Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer. Brit J Caner 114:138–145
Lopez-Riquelme N, Minguela A, Villar-Permuy F et al (2013) Imaging cytometry for counting circulating tumor cells: comparative analysis of the Cell Search vs ImageStream systems. Apmis 121:1139–1143
Min H, Jo SM, Kim HS (2015) Efficient capture and simple quantification of circulating tumor cells using quantum dots and magnetic beads. Small 11:2536–2542
Morimoto A, Mogami T, Watanabe M et al (2015) High-density dielectrophoretic microwell array for detection, capture, and single-cell analysis of rare tumor cells in peripheral blood. PLoS ONE 10:e0130418
Nagrath S, Sequist LV, Maheswaran S et al (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450:1235–1239
Norton L, Massague J (2006) Is cancer a disease of self-seeding? Nat Med 12:875–878
Stott SL, Hsu CH, Tsukrov DI et al (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA 107:18392–18397
Sun JS, Li M, Liu C et al (2012) Double spiral microchannel for label-free tumor cell separation and enrichment. Lab Chip 12:3952–3960
Tang YD, Shi J, Li SS et al (2014) Microfluidic device with integrated microfilter of conical-shaped holes for high efficiency and high purity capture of circulating tumor cells. Sci Rep UK 4:6052
Tsai WS, Chen JS, Shao HJ et al (2016) Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep UK 6:24517
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
van de Stolpe A, Pantel K, Sleijfer S et al (2011) Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res 71:5955–5960
Wang LX, Asghar W, Demirci U et al (2013) Nanostructured substrates for isolation of circulating tumor cells. Nano Today 8:374–387
Wang J, Lu W, Tang C et al (2015) Label-free isolation and mrna detection of circulating tumor cells from patients with metastatic lung cancer for disease diagnosis and monitoring therapeutic efficacy. Anal Chem 87:11893–11900
Warkiani ME, Khoo BL, Wu LD et al (2016) Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat Protoc 11:134–148
Xue P, Ye K, Gao J et al (2014) Isolation and elution of Hep3B circulating tumor cells using a dual-functional herringbone chip. Microfluidics Nanofluidics 16:605–612
Yamashita T, Ji J, Budhu A et al (2009) EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136:1012–1024
Yeo T, Tan SJ, Lim CL et al (2016) Microfluidic enrichment for the single cell analysis of circulating tumor cells. Sci Rep UK 6:22076
Yu M, Bardia A, Aceto N et al (2014) Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345:216–220
Zetter BR (1998) Angiogenesis and tumor metastasis. Annu Rev Med 49:407–424
Zhang FL, Jiang Y, Liu X et al (2016) Hierarchical nanowire arrays as three-dimensional fractal nanobiointerfaces for high efficient capture of cancer cells. Nano Lett 16:766–772
Acknowledgement
P. X. is grateful to the start-up Grant from Southwest University (SWU116032). Y. K. acknowledges the Fundamental Research Funds for the Central Universities (SWU115059, SWU115058, XDJK2016C004, and XDJK2016A010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Xue and Lei Zhang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xue, P., Zhang, L., Guo, J. et al. Isolation and retrieval of circulating tumor cells on a microchip with double parallel layers of herringbone structure. Microfluid Nanofluid 20, 169 (2016). https://doi.org/10.1007/s10404-016-1834-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-016-1834-y