Abstract
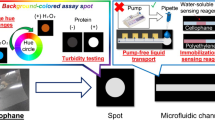
Cotton fabric is proposed as an alternative material for low-cost point-of-care devices. Cotton fabrics are vastly available, low cost and flexible. Simple wax patterning method was applied to create hydrophilic channels in cotton fabric. Three-dimensional (3D) colorimetric microfluidic device was made by folding 2D pattern along certain predefined lines. Three-dimensional devices show better mixing uniformity between reagents and analyte across the detection zones. On-chip colorimetric calibration is also proposed by putting predefined serially diluted samples next to the detection zones. Multiple assays can be integrated within a small surface area by stacking layers of individual assay device separated by a wax-impregnated fabric. We were able to detect glucose, nitrite and protein having concentration as low as 0.5 mM, 30 μM and 0.8 mg/mL, respectively, by bare eyes. Results of the assays from an unknown analyte sample and precalibrated serially diluted sample standards were displayed in a side-by-side configuration, and the interference of each analyte on the other reaction zones was investigated. These results are better than if the detection is merely taken from the calibration curve without integrated standard calibration. The mechanical durability, robustness and flexibility of 3D microfluidic cloth-based analytical device (μCAD) also make it easily embeddable to daily wearable product. We demonstrated multiple single-step qualitative assays using embedded 3D μCAD and propose a new concept of “point-of-sampling diagnostic”.







Similar content being viewed by others
References
Abbott GM, Grosberg P (1966) Measurement of fabric stiffness and hysteresis in bending. Text Res J 36(10):928–930. doi:10.1177/004051756603601012
Abbott GM, Grosberg P, Leaf GAV (1971) The mechanical properties of woven fabrics: Part VII: the hysteresis during bending of woven fabrics. Text Res J 41(4):345–358. doi:10.1177/004051757104100411
Bagherbaigi S, Corcoles EP, Wicaksono DHB (2014) Cotton fabric as an immobilization matrix for low-cost and quick colorimetric enzyme-linked immunosorbent assay (ELISA). Anal Methods. doi:10.1039/c4ay01071j
Ballerini D, Li X, Shen W (2011) An inexpensive thread-based system for simple and rapid blood grouping. Anal Bioanal Chem 399(5):1869–1875. doi:10.1007/s00216-010-4588-5
Behre B (1961) Mechanical properties of textile fabrics Part I: shearing. Text Res J 31(2):87–93. doi:10.1177/004051756103100201
Belfer N (1992) Batik and tie dye techniques. Dover, Mineola
Bhandari P, Narahari T, Dendukuri D (2011) ‘Fab-Chips’: a versatile, fabric-based platform for low-cost, rapid and multiplexed diagnostics. Lab Chip 11(15):2493–2499
Biermann CJ (1996) Handbook of pulping and papermaking. Academic Press, New York
Blicharz TM, Rissin DM, Bowden M, Hayman RB, DiCesare C, Bhatia JS, Grand-Pierre N, Siqueira WL, Helmerhorst EJ, Loscalzo J, Oppenheim FG, Walt DR (2008) Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: a proof of principle. Clin Chem 54(9):1473–1480. doi:10.1373/clinchem.2008.105320
Bruzewicz DA, Reches M, Whitesides GM (2008) Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal Chem 80(9):3387–3392. doi:10.1021/ac702605a
Bryan NS, Grisham MB (2008) Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43(5):645–657
Burch PE (2006) All about hand dyeing. Paula E. Burch. http://www.pburch.net/dyeing.shtml. Accessed 28 May 2014
Carnali JO, Kotkin CA (1993) Determination of the capillary nature of simple woven textiles. J Colloid Interface Sci 159(2):319–323. doi:10.1006/jcis.1993.1329
Carrilho E, Martinez AW, Whitesides GM (2009a) Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem 81(16):7091–7095. doi:10.1021/ac901071p
Carrilho E, Phillips ST, Vella SJ, Martinez AW, Whitesides GM (2009b) Paper microzone plates. Anal Chem 81(15):5990–5998. doi:10.1021/ac900847g
Cheng C-M, Martinez AW, Gong J, Mace CR, Phillips ST, Carrilho E, Mirica KA, Whitesides GM (2010) Paper-based ELISA. Angew Chem Int Ed 49(28):4771–4774. doi:10.1002/anie.201001005
Chibowski E, Gonzalez-Caballero F (1993) Theory and practice of thin-layer wicking. Langmuir 9(1):330–340. doi:10.1021/la00025a062
Chuang M-C, Windmiller JR, Santhosh P, Ramírez GV, Galik M, Chou T-Y, Wang J (2010) Textile-based electrochemical sensing: effect of fabric substrate and detection of nitroaromatic explosives. Electroanalysis 22(21):2511–2518. doi:10.1002/elan.201000434
Chutipongtanate S, Thongboonkerd V (2010) Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal Biochem 402(1):110–112
Clark DB, Miller B (1978) Liquid transport through fabrics; wetting and steady-state flow: Part II: fabric wetting. Text Res J 48(5):256–260. doi:10.1177/004051757804800503
Das B, Das A, Kothari VK, Fangueiro R (2011a) Development of mathematical model to predict vertical wicking behaviour. Part I: flow through yarn. J Text Inst 102(11):957–970. doi:10.1080/00405000.2010.529281
Das B, Das A, Kothari VK, Fangueiro R (2011b) Mathematical model to predict vertical wicking behaviour. Part II: flow through woven fabric. J Text Inst 102(11):971–981. doi:10.1080/00405000.2010.529282
Dungchai W, Chailapakul O, Henry CS (2009) Electrochemical detection for paper-based microfluidics. Anal Chem 81(14):5821–5826. doi:10.1021/ac9007573
Dungchai W, Chailapakul O, Henry CS (2010) Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta 674(2):227–233. doi:10.1016/j.aca.2010.06.019
Ganneboyina SR, Ghatak A (2012) Generation of air–water two-phase flow patterns by altering the helix angle in triple helical microchannels. Ind Eng Chem Res 51(27):9356–9364. doi:10.1021/ie201249g
Grosberg P (1966) The mechanical properties of woven fabrics Part II: the bending of woven fabrics. Text Res J 36(3):205–211. doi:10.1177/004051756603600301
Grosberg P, Park BJ (1966) The mechanical properties of woven fabrics: Part V: the initial modulus and the frictional restraint in shearing of plain weave fabrics. Text Res J 36(5):420–431. doi:10.1177/004051756603600505
Grosberg P, Leaf GAV, Park BJ (1968) The mechanical properties of woven fabrics: Part VI: the elastic shear modulus of plain-weave fabrics. Text Res J 38(11):1085–1100. doi:10.1177/004051756803801102
Hohenberger EF, Kimling H (2008) Compendium urinalysis: urinalysis with test strips, vol 1. Roche Diagnostics GmbH, Mannheim
Hollies NRS, Kaessinger MM, Bogaty H (1956) Water transport mechanisms in textile materials1 Part I: the role of yarn roughness in capillary-type penetration. Text Res J 26(11):829–835. doi:10.1177/004051755602601102
Hollies NRS, Kaessinger MM, Watson BS, Bogaty H (1957) Water transport mechanisms in textile materials: Part II: capillary-type penetration in yarns and fabrics. Text Res J 27(1):8–13. doi:10.1177/004051755702700102
Hönes J, Müller P, Surridge N (2008) The technology behind glucose meters: test strips. Diabetes Technol Ther 10(S1):S10–S26
Hsieh Y-L (1995) Liquid transport in fabric structures. Text Res J 65(5):299–307. doi:10.1177/004051759506500508
Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT (2012) Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative loop-mediated isothermal amplification. Angew Chem Int Ed 51(20):4896–4900. doi:10.1002/anie.201109115
Jones SW, Thomas OM, Aref H (1989) Chaotic advection by laminar flow in a twisted pipe. J Fluid Mech 209:335–357
Jung H-C, Moon J-H, Baek D-H, Lee J-H, Choi Y-Y, Hong J-S, Lee S-H (2012) CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. Biomed Eng IEEE Trans On 59(5):1472–1479
Kadolph SJ (2010) Textiles, 11th edn. Prentice Hall, Upper Saddle River
Karimpil JJ, Melo JS, D’Souza SF (2012) Immobilization of lipase on cotton cloth using the layer-by-layer self-assembly technique. Int J Biol Macromol 50(1):300–302. doi:10.1016/j.ijbiomac.2011.10.019
Lewis M (2012) Agarose gel electrophoresis (basic method). http://www.methodbook.net/dna/agarogel.html. Accessed 29 May 2012
Li X, Tian J, Nguyen T, Shen W (2008) Paper-based microfluidic devices by plasma treatment. Anal Chem 80(23):9131–9134. doi:10.1021/ac801729t
Li X, Tian J, Shen W (2010a) Quantitative biomarker assay with microfluidic paper-based analytical devices. Anal Bioanal Chem 396(1):495–501. doi:10.1007/s00216-009-3195-9
Li X, Tian J, Shen W (2010b) Thread as a versatile material for low-cost microfluidic diagnostics. ACS Appl Mater Interfaces 2(1):1–6. doi:10.1021/am9006148
Liu H, Crooks RM (2011) Three-dimensional paper microfluidic devices assembled using the principles of origami. J Am Chem Soc 133(44):17564–17566. doi:10.1021/ja2071779
Liu XY, Cheng CM, Martinez AW, Mirica KA, Li XJ, Phillips ST, Mascareñas M, Whitesides GM (2011) A portable microfluidic paper-based device for ELISA. In: 2011 IEEE 24th international conference on micro electro mechanical systems (MEMS), 23–27 Jan 2011, pp 75–78
Lomov SV, Huysmans G, Verpoest I (2001) Hierarchy of textile structures and architecture of fabric geometric models. Text Res J 71(6):534–543. doi:10.1177/004051750107100611
Lu Y, Shi W, Jiang L, Qin J, Lin B (2009) Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 30(9):1497–1500. doi:10.1002/elps.200800563
Mabey D, Peeling RW, Ustianowski A, Perkins MD (2004) Tropical infectious diseases: diagnostics for the developing world. Nat Rev Microbiol 2(3):231–240
Malon RSP, Chua KY, Wicaksono DHB, Corcoles EP (2014) Cotton fabric-based electrochemical device for lactate measurement in saliva. Analyst 139(12):3009–3016. doi:10.1039/c4an00201f
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed 46(8):1318–1320. doi:10.1002/anie.200603817
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM (2008a) Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80(10):3699–3707. doi:10.1021/ac800112r
Martinez AW, Phillips ST, Whitesides GM (2008b) Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci 105(50):19606–19611. doi:10.1073/pnas.0810903105
Martinez AW, Phillips ST, Nie Z, Cheng C-M, Carrilho E, Wiley BJ, Whitesides GM (2010a) Programmable diagnostic devices made from paper and tape. Lab Chip 10(19):2499–2504
Martinez AW, Phillips ST, Whitesides GM, Carrilho E (2010b) Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82(1):3–10. doi:10.1021/ac9013989
Masoodi R, Pillai KM (2010) Darcy’s law-based model for wicking in paper-like swelling porous media. AIChE J 56(9):2257–2267. doi:10.1002/aic.12163
Muhonen P (2013) Brittleness of paper. Lappeenranta University of Technology, Lappeenranta
Nickerson RF (1940) Cotton fibers constitution, structure, and mechanical properties. Ind Eng Chem 32(11):1454–1462. doi:10.1021/ie50371a012
Nie Z, Ca Nijhuis, Gong J, Chen X, Kumachev A, Martinez AW, Narovlyansky M, Whitesides GM (2010) Electrochemical sensing in paper-based microfluidic devices. Lab Chip 10(4):477–483
Nilghaz A, Wicaksono DHB, Majid FAA ( 2011a) Batik-inspired wax patterning for cloth-based microfluidic device. In: 2011 2nd international conference on instrumentation control and automation (ICA), 15–17 Nov 2011, pp 82–86. doi:10.1109/ica.2011.6130134
Nilghaz A, Wicaksono DHB, Supriyanto E (2011b) Simultaneous multiple assays on microfluidic cloth-based analytical devices. In: 2011 2nd international conference on instrumentation control and automation (ICA), 15–17 Nov 2011, pp 266–268. doi:10.1109/ica.2011.6130169
Nilghaz A, Wicaksono DHB, Gustiono D, Abdul Majid FA, Supriyanto E, Abdul Kadir MR (2012) Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. Lab Chip 12(1):209–218
Nilghaz A, Ballerini DR, Fang X-Y, Shen W (2014) Semiquantitative analysis on microfluidic thread-based analytical devices by ruler. Sens Actuators B Chem 191:586–594. doi:10.1016/j.snb.2013.10.023
Nyoni AB, Brook D (2006) Wicking mechanisms in yarns—the key to fabric wicking performance. J Text Inst 97(2):119–128. doi:10.1533/joti.2005.0128
Parikesit GOF, Prasetia F, Pribadi GA, Simbolon DC, Pradhana GY, Prastowo AR, Gunawan A, Suryopratomo K, Kusumaningtyas I (2012) Textile-based microfluidics: modulated wetting, mixing, sorting, and energy harvesting. J Text Inst 103(10):1077–1087. doi:10.1080/00405000.2012.660756
Park CH, Kang YK, Im SS (2004) Biodegradability of cellulose fabrics. J Appl Polym Sci 94(1):248–253. doi:10.1002/app.20879
Reches M, Mirica KA, Dasgupta R, Dickey MD, Butte MJ, Whitesides GM (2010) Thread as a matrix for biomedical assays. ACS Appl Mater Interfaces 2(6):1722–1728. doi:10.1021/am1002266
Sackmann EK, Fulton AL, Beebe DJ (2014) The present and future role of microfluidics in biomedical research. Nature 507(7491):181–189. doi:10.1038/nature13118
Şahinbaşkan BY, Kahraman MV (2011) Desizing of untreated cotton fabric with the conventional and ultrasonic bath procedures by immobilized and native α-amylase. Starch-Stärke 63(3):154–159. doi:10.1002/star.201000109
Sánchez-Patán F, Anchuelo R, Aller M-A, Vara E, García C, Nava M-P, Arias J (2008) Chronic prehepatic portal hypertension in the rat: is it a type of metabolic inflammatory syndrome? Lipids Health Dis 7(4):1–10
Sharma H, Nguyen D, Chen A, Lew V, Khine M (2010) Unconventional low-cost fabrication and patterning techniques for point of care diagnostics. Ann Biomed Eng. doi:10.1007/s10439-010-0213-1
Strasinger SK, Di Lorenzo MS (2014) Urinalysis and body fluids. FA Davis, Philadelphia
Sumi S, Mathai A, Radhakrishnan VV (2009) Dot-immunobinding assay. In: Kurien BT, Scofield RH (eds) Protein blotting and detection. Methods in molecular biology, vol 536. Humana Press, New York, pp 89–93. doi:10.1007/978-1-59745-542-8_11
Tietz NW (1995) Clinical guide to laboratory tests. W. B. Saunders Company, Philadelphia
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J Chromatogr B Anal Technol Biomed Life Sci 851(1–2):51–70
Wang W, Wu W-Y, Zhu J-J (2010) Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. J Chromatogr A 1217(24):3896–3899
Windmiller JR, Wang J (2013) Wearable electrochemical sensors and biosensors: a review. Electroanalysis 25(1):29–46. doi:10.1002/elan.201200349
Windmiller JR, Bandodkar AJ, Parkhomovsky S, Wang J (2012) Stamp transfer electrodes for electrochemical sensing on non-planar and oversized surfaces. Analyst 137(7):1570–1575. doi:10.1039/c2an35041f
Yang M, Sun S, Kostov Y, Rasooly A (2011) A simple 96-well microfluidic chip combined with visual and densitometry detection for resource-poor point of care testing. Sens Actuators B Chem 153(1):176–181
Yu B, James Lee L (2000) A simplified in-plane permeability model for textile fabrics. Polym Compos 21(5):660–685. doi:10.1002/pc.10221
Zhou G, Mao X, Juncker D (2012) Immunochromatographic assay on thread. Anal Chem 84(18):7736–7743. doi:10.1021/ac301082d
Acknowledgments
The project is funded by Universiti Teknologi Malaysia (UTM) through Tier-1 Research University (RU) Grant under Vot. No. 01H65, 03H30, 05H32, and A.N. was also supported by UTM through Foreign Academic Visitor Fund (FAVF) Vot. No. R.J130000.7736.4D004. The project is also supported by Ministry of Education Malaysia (MoE) under Fundamental Research Grant (FRGS) Vot. No. 4F328. We thank UTM’s Research Management Centre (RMC) for managing the grants. We would like to thank Prof. Ir. Dr. M. Rafiq Abd. Kader for his support at Mediteg Lab, Faculty of Biosciences and Medical Engineering, UTM. We would like to thank Mr. Syed Mustafa Syed Azman for technical helps during the experiments. We acknowledge the help of Mr. Hairol Akmal Jawahir for videotaping the experimental procedures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 4 (MP4 57676 kb)
Supplementary material 5 (MP4 70309 kb)
Supplementary material 6 (MP4 35384 kb)
Supplementary material 7 (MP4 46309 kb)
Supplementary material 8 (MP4 55289 kb)
Supplementary material 9 (MP4 29406 kb)
Supplementary material 10 (MP4 60192 kb)
Supplementary material 11 (MP4 41284 kb)
Supplementary material 12 (MP4 53864 kb)
Supplementary material 13 (MP4 43533 kb)
Rights and permissions
About this article
Cite this article
Nilghaz, A., Bagherbaigi, S., Lam, C.L. et al. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (μCAD) for effective point-of-care diagnostic. Microfluid Nanofluid 19, 317–333 (2015). https://doi.org/10.1007/s10404-015-1545-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1545-9