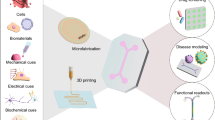
Abstract
Engineering living systems on chips is an emerging direction with a goal to mimic physiologically accurate biological functions that can be applied to a diversity of applications, such as reliable in vitro drug-screening systems for reducing the need for animal testing. Taking advantages of creative platforms from electromechanical systems technology and from advanced biomaterials to mimic 3D extracellular matrix, these approaches to recapitulating organ-level structures and functions may bring unprecedented benefits to clinical translation of nanomedicines in the pharmaceutical and biomedical industries and to advanced tissue engineering for regenerative medicine. In this review, we discuss recent progress on the engineering of living systems on chips and highlight advanced technologies that integrate a variety of physiological cues including mechanical, chemical, and electrical signals with precise spatiotemporal controls. We also discuss current challenges and future directions of these approaches, analyzing the benefits of continued research in this field.






Similar content being viewed by others
References
Altomare L, Riehle M, Gadegaard N, Tanzi MC, Fare S (2010) Microcontact printing of fibronectin on a biodegradable polymeric surface for skeletal muscle cell orientation. Int J Artif Organs 33(8):535–543
Balachandran K, Alford PW, Wylie-Sears J, Goss JA, Grosberg A, Bischoff J, Aikawa E, Levine RA, Parker KK (2011) Cyclic strain induces dual-mode endothelial-mesenchymal transformation of cardiac valve. Proc Natl Acad Sci USA 108(50):19943–19948. doi:10.1073/pnas.1106954108
Bellin RM, Kubicek JD, Frigault MJ, Kamien AJ, Steward RL Jr, Barnes HM, Digiacomo MB, Duncan LJ, Edgerly CK, Morse EM, Park CY, Fredberg JJ, Cheng CM, LeDuc PR (2009) Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc Natl Acad Sci USA 106(52):22102–22107. doi:10.1073/pnas.0902639106
Bonvin C, Overney J, Shieh AC, Dixon JB, Swartz MA (2010) A multichamber fluidic device for 3D cultures under interstitial flow with live imaging: development, characterization, and applications. Biotechnol Bioeng 105(5):982–991. doi:10.1002/bit.22608
Chaudhuri P, Harfouche R, Soni S, Hentschel DM, Sengupta S (2010) Shape effect of carbon nanovectors on angiogenesis. ACS Nano 4(1):574–582
Cheng W, Klauke N, Sedgwick H, Smith GL, Cooper JM (2006) Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform. Lab Chip 6(11):1424–1431. doi:10.1039/b608202e
Cheng W, Klauke N, Smith G, Cooper JM (2010) Microfluidic cell arrays for metabolic monitoring of stimulated cardiomyocytes. Electrophoresis 31(8):1405–1413. doi:10.1002/elps.200900579
Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD (2009) Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9(2):269–275
Dixon JB, Raghunathan S, Swartz MA (2009) A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng 103(6):1224–1235. doi:10.1002/bit.22337
El-Ali J, Sorger PK, Jensen KF (2006) Cells on chips. Nature 442(7101):403–411. doi:10.1038/nature05063
Esch MB, King TL, Shuler ML (2011) The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 13:55–72
Esch MB, Sung JH, Yang J, Yu C, Yu J, March JC, Shuler ML (2012) On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed Microdevices 14(5):895–906. doi:10.1007/s10544-012-9669-0
Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK (2007) Muscular thin films for building actuators and powering devices. Science 317(5843):1366–1370. doi:10.1126/science.1146885
Feinberg AW, Alford PW, Jin H, Ripplinger CM, Werdich AA, Sheehy SP, Grosberg A, Parker KK (2012) Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 33(23):5732–5741. doi:10.1016/j.biomaterials.2012.04.043
Fisher RJ, Peattie RA (2007) Controlling tissue microenvironments: biomimetics, transport phenomena, and reacting systems. Adv Biochem Eng Biotechnol 103:1–73
Fujie T, Ahadian S, Liu H, Chang H, Ostrovidov S, Wu H, Bae H, Nakajima K, Kaji H, Khademhosseini A (2013) Engineered nanomembranes for directing cellular organization toward flexible biodevices. Nano Lett. doi:10.1021/nl401237s
Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A (2012) Biomimetic tissues on a chip for drug discovery. Drug Discov Today 17(3–4):173–181. doi:10.1016/j.drudis.2011.10.029
Giri S, Braumann UD, Giri P, Acikgoz A, Scheibe P, Nieber K, Bader A (2013) Nanostructured self-assembling peptides as a defined extracellular matrix for long-term functional maintenance of primary hepatocytes in a bioartificial liver modular device. Int J Nanomed 8:1525–1539. doi:10.2147/IJN.S33589
Golomb BA, Kwon EK, Koperski S, Evans MA (2009) Amyotrophic lateral sclerosis-like conditions in possible association with cholesterol-lowering drugs: an analysis of patient reports to the University of California, San Diego (UCSD) Statin Effects Study. Drug Saf Int J Med Toxicol Drug Exp 32(8):649–661. doi:10.2165/00002018-200932080-00004
Grosberg A, Alford PW, McCain ML, Parker KK (2011) Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 11(24):4165–4173
Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK (2012) Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods 65(3):126–135. doi:10.1016/j.vascn.2012.04.001
Günther A, Yasotharan S, Vagaon A, Lochovsky C, Pinto S, Yang J, Lau C, Voigtlaender-Bolz J, Bolz SS (2010) A microfluidic platform for probing small artery structure and function. Lab Chip 10(18):2341–2349
Haessler U, Teo JC, Foretay D, Renaud P, Swartz MA (2012) Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr Biol Quant Biosci Nano Macro 4(4):401–409. doi:10.1039/c1ib00128k
Hamdi Kural M, Lawrence Billiar K (2013) Regulating tension in three-dimensional culture environments. Exp Cell Res. doi:10.1016/j.yexcr.2013.06.019
Hattersley SM, Greenman J, Haswell SJ (2011) Study of ethanol induced toxicity in liver explants using microfluidic devices. Biomed Microdevices 13(6):1005–1014
Hattersley SM, Sylvester DC, Dyer CE, Stafford ND, Haswell SJ, Greenman J (2012) A microfluidic system for testing the responses of head and neck squamous cell carcinoma tissue biopsies to treatment with chemotherapy drugs. Ann Biomed Eng 40(6):1277–1288
Hood JL, Pan H, Lanza GM, Wickline SA (2009) Consortium for Translational Research in Advanced I, Nanomedicine (2009) Paracrine induction of endothelium by tumor exosomes. Lab Investig J Tech Methods Pathol 89(11):1317–1328. doi:10.1038/labinvest.2009.94
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328(5986):1662–1668
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21(12):745–754. doi:10.1016/j.tcb.2011.09.005
Hutzler M, Fromherz P (2004) Silicon chip with capacitors and transistors for interfacing organotypic brain slice of rat hippocampus. Eur J Neurosci 19(8):2231–2238
Irimia D, Toner M (2009) Spontaneous migration of cancer cells under conditions of mechanical confinement. Integ Biol Quant Biosci Nano Macro 1(8–9):506–512. doi:10.1039/b908595e
Kaji H, Ishibashi T, Nagamine K, Kanzaki M, Nishizawa M (2010) Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials 31(27):6981–6986. doi:10.1016/j.biomaterials.2010.05.071
Kam NW, Jan E, Kotov NA (2009) Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett 9(1):273–278
Kim J, Hegde M, Jayaraman A (2010) Co-culture of epithelial cells and bacteria for investigating host-pathogen interactions. Lab Chip 10(1):43–50. doi:10.1039/b911367c
Kim Y, Joshi SD, Messner WC, LeDuc PR, Davidson LA (2011) Detection of dynamic spatiotemporal response to periodic chemical stimulation in a Xenopus embryonic tissue. PLoS ONE 6(1):e14624. doi:10.1371/journal.pone.0014624
Kim HJ, Huh D, Hamilton G, Ingber DE (2012) Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12(12):2165–2174. doi:10.1039/c2lc40074j
Kim S, Lee H, Chung M, Jeon NL (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13(8):1489–1500. doi:10.1039/c3lc41320a
Kuczenski B, Ruder WC, Messner WC, LeDuc PR (2009) Probing cellular dynamics with a chemical signal generator. PLoS ONE 4(3):e4847
Kusunose J, Zhang H, Gagnon MK, Pan T, Simon SI, Ferrara KW (2013) Microfluidic system for facilitated quantification of nanoparticle accumulation to cells under laminar flow. Ann Biomed Eng 41(1):89–99. doi:10.1007/s10439-012-0634-0
Kuttenberger J, Polska E, Schaefer BM (2013) A novel three-dimensional bone chip organ culture. Clin Oral Investig 17(6):1547–1555. doi:10.1007/s00784-012-0833-y
Lee DW, Lee MY, Ku B, Yi SH, Ryu JH, Jeon R, Yang M (2013a) Application of the DataChip/MetaChip technology for the evaluation of ajoene toxicity in vitro. Arch Toxicol. doi:10.1007/s00204-013-1102-9
Lee SA, No DY, Kang E, Ju J, Kim DS, Lee SH (2013b) Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. doi:10.1039/c3lc50197c
Li L, Liu W, Wang J, Tu Q, Liu R, Wang J (2010) Lectin-aided separation of circulating tumor cells and assay of their response to an anticancer drug in an integrated microfluidic device. Electrophoresis 31(18):3159–3166. doi:10.1002/elps.201000139
Lu J, Barrios CA, Dickson AR, Nourse JL, Lee AP, Flanagan LA (2012) Advancing practical usage of microtechnology: a study of the functional consequences of dielectrophoresis on neural stem cells. Integ Biol Quant Biosci Nano Macro 4(10):1223–1236. doi:10.1039/c2ib20171b
Mao S, Gao D, Liu W, Wei H, Lin JM (2012) Imitation of drug metabolism in human liver and cytotoxicity assay using a microfluidic device coupled to mass spectrometric detection. Lab Chip 12(1):219–226. doi:10.1039/c1lc20678h
Materne EM, Tonevitsky AG, Marx U (2013) Chip-based liver equivalents for toxicity testing—organotypicalness versus cost-efficient high throughput. Lab Chip. doi:10.1039/c3lc50240f
McKellar AE, Hendry AP (2009) How humans differ from other animals in their levels of morphological variation. PLoS ONE 4(9):e6876. doi:10.1371/journal.pone.0006876
Meyvantsson I, Beebe DJ (2008) Cell culture models in microfluidic systems. Annu Rev Anal Chem (Palo Alto Calif) 1:423–449. doi:10.1146/annurev.anchem.1.031207.113042
Moraes C, Mehta G, Lesher-Perez SC, Takayama S (2012) Organs-on-a-chip: a focus on compartmentalized microdevices. Ann Biomed Eng 40(6):1211–1227
Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S (2013) On being the right size: scaling effects in designing a human-on-a-chip. Integ Biol Quant Biosci Nano Macro 5(9):1149–1161. doi:10.1039/c3ib40040a
Munson JM, Bellamkonda RV, Swartz MA (2013) Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res 73(5):1536–1546. doi:10.1158/0008-5472.CAN-12-2838
Nguyen DHT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS (2013) Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. PNAS 110(17):6712–6717
Nipper ME, Dixon JB (2011) Engineering the lymphatic system. Cardiovasc Eng Technol 2(4):296–308. doi:10.1007/s13239-011-0054-6
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845
Patel V, Martin D, Malhotra R, Marsh CA, Doci CL, Veenstra TD, Nathan CA, Sinha UK, Singh B, Molinolo AA, Rusling JF, Gutkind JS (2013) DSG3 as a biomarker for the ultrasensitive detection of occult lymph node metastasis in oral cancer using nanostructured immunoarrays. Oral Oncol 49(2):93–101. doi:10.1016/j.oraloncology.2012.08.001
Peterman MC, Mehenti NZ, Bilbao KV, Lee CJ, Leng T, Noolandi J, Bent SF, Blumenkranz MS, Fishman HA (2003) The artificial synapse chip: a flexible retinal interface based on directed retinal cell growth and neurotransmitter stimulation. Artif Organs 27(11):975–985
Prot JM, Bunescu A, Elena-Herrmann B, Aninat C, Snouber LC, Griscom L, Razan F, Bois FY, Legallais C, Brochot C, Corlu A, Dumas ME, Leclerc E (2012) Predictive toxicology using systemic biology and liver microfluidic “on chip” approaches: application to acetaminophen injury. Toxicol Appl Pharmacol 259(3):270–280. doi:10.1016/j.taap.2011.12.017
Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, Kamm RD, Chen CS, Asada HH (2012) Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12(23):4976–4985. doi:10.1039/c2lc40338b
Shein M, Greenbaum A, Gabay T, Sorkin R, David-Pur M, Ben-Jacob E, Hanein Y (2009) Engineered neuronal circuits shaped and interfaced with carbon nanotube microelectrode arrays. Biomed Microdevices 11(2):495–501
Shieh AC, Rozansky HA, Hinz B, Swartz MA (2011) Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res 71(3):790–800. doi:10.1158/0008-5472.CAN-10-1513
Shih MC, Tseng SH, Weng YS, Chu IM, Liu CH (2013) A microfluidic device mimicking acinar concentration gradients across the liver acinus. Biomed Microdevices. doi:10.1007/s10544-013-9762-z
Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, Sudo R, Kamm RD, Chung S (2011) In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11(13):2175–2181. doi:10.1039/c1lc20039a
Sun Y, Duffy R, Lee A, Feinberg AW (2013) Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater 9(8):7885–7894. doi:10.1016/j.actbio.2013.04.036
Sung JH, Shuler ML (2009) A micro cell culture analog (mu CCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 9(10):1385–1394. doi:10.1039/b901377f
Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, Shuler ML (2013) Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 13(7):1201–1212. doi:10.1039/c3lc41017j
Suwanpayak N, Jalil MA, Aziz MS, Ismail FD, Ali J, Yupapin PP (2011) Blood cleaner on-chip design for artificial human kidney manipulation. Int J Nanomed 6:957–964. doi:10.2147/IJN.S19077
Tanaka Y, Morishima K, Shimizu T, Kikuchi A, Yamato M, Okano T, Kitamori T (2006) Demonstration of a PDMS-based bio-microactuator using cultured cardiomyocytes to drive polymer micropillars. Lab Chip 6(2):230–235. doi:10.1039/b512099c
van der Meer AD, van den Berg A (2012) Organs-on-chips: breaking the in vitro impasse. Integ Biol Quant Biosci Nano Macro 4(5):461–470
van der Meer AD, Orlova VV, Ten Dijke P, van den Berg A, Mummery CL (2013) Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. doi:10.1039/c3lc50435b
Wagner I, Materne EM, Brincker S, Sussbier U, Fradrich C, Busek M, Sonntag F, Sakharov DA, Trushkin EV, Tonevitsky AG, Lauster R, Marx U (2013) A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. doi:10.1039/c3lc50234a
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260(5111):1124–1127
Wikswo J, Block F III, Cliffel D, Goodwin C, Marasco C, Markov D, McLean D, McLean J, McKenzie J, Reiserer R, Samson P, Schaffer D, Seale K, Sherrod S (2013) Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans Biomed Eng 60(3):682–690. doi:10.1109/TBME.2013.2244891
Williamson A, Singh S, Fernekorn U, Schober A (2013) The future of the patient-specific Body-on-a-chip. Lab Chip. doi:10.1039/c3lc50237f
Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL (2012) In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 12(16):2815–2822. doi:10.1039/c2lc40131b
Zhao Y, Zhang X (2006) Cellular mechanics study in cardiac myocytes using PDMS pillars array. Sens Actuators, A 125(2):398–404
Zhao Y, Lim CC, Sawyer DB, Liao R, Zhang X (2007) Simultaneous orientation and cellular force measurements in adult cardiac myocytes using three-dimensional polymeric microstructures. Cell Motil Cytoskelet 64(9):718–725. doi:10.1002/cm.20218
Zheng W, Jiang B, Wang D, Zhang W, Wang Z, Jiang X (2012) A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 12(18):3441–3450. doi:10.1039/c2lc40173h
Zhou J, Niklason LE (2012) Microfluidic artificial “vessels” for dynamic mechanical stimulation of mesenchymal stem cells. Integ Biol Quant Biosci Nano Macro 4(12):1487–1497. doi:10.1039/c2ib00171c
Acknowledgments
We thank Georgia Institute of Technology for the startup resources. This work was supported in part by the National Science Foundation, NSF Grant Numbers (CMMI-1100430, CMMI-1160840, CPS-1135850), and the Air Force Office of Scientific Research (FA9550-13-1-01 08).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yoshitaka Sei and Kyle Justus have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sei, Y., Justus, K., LeDuc, P. et al. Engineering living systems on chips: from cells to human on chips. Microfluid Nanofluid 16, 907–920 (2014). https://doi.org/10.1007/s10404-014-1341-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1341-y