Abstract
Background and purpose
Flow velocity oscillation rate (FVOR) of the renal interlobar vein has been reported to be decreased in patients with urinary obstruction or diabetic nephropathy, and increased in those with hypertension during pregnancy. To clarify the clinical role of the renal interlobar venous FVOR, we investigated the flow velocity patterns of the renal vessels in patients with hypertension (HT) and/or diabetes (DM).
Methods and results
Pulsed-wave Doppler sonography was performed in 34 patients: 15 with HT, 10 with DM, and nine with both HT and DM (HT-DM). Each FVOR of the right and left interlobar veins was closely and positively correlated with the ipsilateral interlobar arterial resistive index (RI), especially in the HT group, but not with the estimated glomerular filtration rate. The right interlobar venous FVOR was decreased in the DM and HT-DM groups compared to the HT group.
Conclusion
The renal interlobar venous FVOR is strongly influenced by the arterial RI in HT patients, and is reduced in DM patients without an obvious relationship with diabetic nephropathy. These findings should be noted for the clinical application of renal interlobar venous flow analysis.





Similar content being viewed by others
Change history
23 October 2017
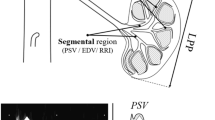
In the original publication of this paper the legend of Fig. 1 should read as: Fig. 1 Pulsed Doppler flow velocity recordings of the aorta (a), right renal artery (b), right renal interlobar artery (c), inferior vena cava (d), right renal vein (e), and right renal interlobar vein (f). PSV peak systolic velocity, EDV end-diastolic velocity, V MAX maximum velocity, V MIN minimum velocity
References
Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703.
Nosadini R, Velussi M, Brocco E, et al. Increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. Diabetes. 2006;55:234–9.
Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–7.
Rifkin MD, Needleman L, Pasto ME, et al. Evaluation of renal transplant rejection by duplex Doppler examination: value of the resistive index. Am J Roentgenol. 1987;148:759–62.
Park SJ, Lim JW, Cho BS, et al. Nutcracker syndrome in children with orthostatic proteinuria: diagnosis on the basis of Doppler sonography. J Ultrasound Med. 2002;21:39–45.
Cheon JE, Kim WS, Kim IO, et al. Nutcracker syndrome in children with gross hematuria: Doppler sonographic evaluation of the left renal vein. Pediatr Radiol. 2006;36:682–6.
Fitoz S, Ekim M, Ozoakar ZB, et al. Nutcracker syndrome in children: the role of upright position examination and superior mesenteric artery angle measurement in the diagnosis. J Ultrasound Med. 2007;26:573–80.
Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. Am J Roentgenol. 2002;178:921–5.
Oktar SÖ, Yücel C, Özdemir H, et al. Doppler sonography of renal obstruction: value of venous impedance index measurements. J Ultrasound Med. 2004;23:929–36.
Bateman GA, Giles W, England SL. Renal venous Doppler sonography in preeclampsia. J Ultrasound Med. 2004;23:1607–11.
Gyselaers W, Mesens T, Tomsin K, et al. Maternal renal interlobar vein impedance index is higher in early-than in late-onset preeclampsia. Ultrasound Obstet Gynecol. 2010;36:69–75.
Gyselaers W, Mullens W, Tomsin K, et al. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: a review. Ultrasound Obstet Gynecol. 2011;38:123–9.
Jeong SH, Jung DC, Kim SH, et al. Renal venous doppler ultrasonography in normal subjects and patients with diabetic nephropathy: value of venous impedance index measurements. J Clin Ultrasound. 2011;39:512–8.
Weber MA, Schiffrin EL, White WB, et al. Clinical Practice Guidelines for the Management of Hypertension in the Community A Statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–16.
Karabulut N, Baki Yağci A, Karabulut A. Renal vein Doppler ultrasound of maternal kidneys in normal second and third trimester pregnancy. Br J Radiol. 2003;76:444–7.
Paul AD. The kidney. In: Paul LA, Paul AD, Myron AP, et al., editors. Clinical Doppler ultrasound. 2nd ed. UK: Churchill Livingstone; 2006. p. 185–214.
Rajagopalan B, Friend JA, Stallard T, et al. Blood flow in pulmonary veins: II. The influence of events transmitted from the right and left sides of the heart. Cardiovasc Res. 1979;13:677–83.
Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. J Neurol Neurosurg Psychiatry. 2003;74:7–9.
Morgan WH, Hazelton ML, Yu DY. Retinal venous pulsation: expanding our understanding and use of this enigmatic phenomenon. Prog Retin Eye Res. 2016;55:1–26.
Iida N, Seo Y, Sai S, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients prior to their inclusion in the study.
Conflict of interest
Yusuke Kudo, Taisei Mikami, Mutsumi Nishida, Kazunori Okada, Sanae Kaga, Nobuo Masauzi, Satomi Omotehara, Hitoshi Shibuya, Kaoru Kahata, and Chikara Shimizu do not have any relationship that could lead to a conflict of interest.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10396-017-0835-0.
About this article
Cite this article
Kudo, Y., Mikami, T., Nishida, M. et al. Altered oscillation of Doppler-derived renal and renal interlobar venous flow velocities in hypertensive and diabetic patients. J Med Ultrasonics 44, 305–314 (2017). https://doi.org/10.1007/s10396-017-0770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-017-0770-0