Abstract
Purpose
The objective was to investigate the contributions of mechanical effects due to kinetic force induced by the dynamic behavior of microbubbles and sonochemical effects due to free radicals produced by inertial cavitation to cell membrane damage under sonoporation conditions in which cells with adjacent microbubbles were irradiated with single-shot pulsed ultrasound.
Methods
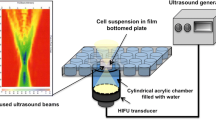
The free radical scavenger cysteamine was used to control the occurrence of sonochemical effects, and the ratios of cells with membrane damage to intact cells were compared in the presence and absence of cysteamine. To determine the optimal dose of cysteamine, free radical production on exposure to burst pulse ultrasound was investigated using KI-starch solutions with different concentrations (0–5 mM) of cysteamine. High-speed observation of the dynamic behavior of Levovist microbubbles during ultrasound exposure was also carried out in the presence and absence of cysteamine, and the difference in the ratios of the maximum bubble diameter to the initial diameter was evaluated. Next, human prostate cancer cells with adjacent Levovist microbubbles were exposed to single-shot pulsed ultrasound with a center frequency of 1 MHz, a peak negative pressure of 1.1 MPa, and a pulse width of 3 μs, and the percentages of cells with membrane damage were evaluated by fluorescent microscopy using propidium iodide in the presence and absence of cysteamine.
Results
It was confirmed that cysteamine at a concentration of 5 mM completely suppressed sonochemical effects without causing a change in the dynamic response of microbubbles to pulsed ultrasound. The percentages of cells with membrane damage in the presence and absence of cysteamine (5 mM) were 10.3% ± 4.1% (n = 13) and 8.7% ± 3.9% (n = 9), respectively. No significant difference was found (P = 0.36).
Conclusion
The results indicate that cell membrane damage induced by single-shot pulsed ultrasound with adjacent microbubbles was due mainly to mechanical effects, not to sonochemical effects.
Similar content being viewed by others
References
Newman CM, Lawrie A, Brisken AF, et al. Ultrasound gene therapy: on the road from concept to reality. Echocardiography 2001;18:339–447.
Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet 2002;27:115–134.
Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther 2002;9:1647–1652.
Mehier-Humbert S, Guy RH. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv Drug Deliv Rev 2005;57:733–753.
Bao S, Thrall BD. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 1997;23: 953–959.
Pislaru SV, Pislaru C, Kinnick RR, et al. Optimization of ultrasound-mediated gene transfer: comparison of contrast agents and ultrasound modalities. Eur Heart J 2003;24:1690–1698.
Miller DL, Quddus J. Lysis and sonoporation of epidermoid and phagocytic monolayer cells by diagnostic ultrasound activation of contrast agent gas bodies. Ultrasound Med Biol 2001;27:1107–1113.
Miller DL, Quddus J. Diagnostic ultrasound-induced membrane damage in phagocytic cells loaded with contrast agent and its relation to Doppler-mode images. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:1094–102.
Kudo N, Miyaoka T, Okada K, et al. Study on mechanism of cell damage caused by microbubbles exposed to ultrasound. In: Proceedings of 2002 IEEE Ultrasonics Symposium, vol 2. Munich: IEEE; 2002. p. 1383–1386.
Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003;423:153–156.
Postema M, van Wamel A, Lancée CT, et al. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound Med Biol 2004;30:827–840.
Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med 1992;13: 247–270.
Korosoglou G, Hardt SE, Bekeredjian R, et al. Ultrasound exposure can increase the membrane permeability of human neutrophil granulocytes containing microbubbles without causing complete cell destruction. Ultrasound Med Biol 2006;32:297–303.
Flynn HG. Generation of transient cavities in liquids by microsecond pulses of ultrasound. J Acoust Soc Am 1982;72:1926–1932.
Crum LA, Fowlkes JB. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature 1986;319:52–54.
Fowlkes JB, Crum LA. Cavitation threshold measurements for microsecond length pulses of ultrasound. J Acoust Soc Am 1988;83:2190–2201.
Kondo T, Nishimura J, Kitagawa H, et al. Optimization of enhancement of therapeutic efficacy of ultrasound frequency-dependent effects on iodine formation from KI-starch solutions and ultrasound-induced killing of rat thymocytes. J Med Ultrasonics 2003; 30:93–101.
Okada K, Kudo N, Niwa K, et al. A basic study on sonoporation with microbubbles exposed to pulsed ultrasound. J Med Ultrasonics 2005;32:3–11.
Kondo T, Yoshii G. Effect of intensity of 1.2 MHz ultrasound on change in DNA synthesis of irradiated mouse L cells. Ultrasound Med Biol 1985;11:113–119.
Miller DL, Williams AR. Bubble cycling as the explanation of the promotion of ultrasonic cavitation in a rotating tube exposure system. Ultrasound Med Biol 1989;15:641–648.
Roots R, Okada S. Estimation of lifetime and diffusion distances of radicals involved in X-ray-induced DNA strand breaks or killing of mammalian cells. Radiat Res 1975;64:306–320.
Lawrie A, Brisken AF, Francis SE, et al. Ultrasound-enhanced transgene expression in vascular cells is not dependent upon cavitation-induced free radicals. Ultrasound Med Biol 2003;29: 1453–1461.
Clarke PR, Hill CR. Physical and chemical aspects of ultrasonic disruption of cells. J Acoust Soc Am 1970;47:649–653.
Young FR. Sonoluminescence from water containing dissolved gases. J Acoust Soc Am 1976;60:100–104.
Kondo T, Misík V, Riesz P. Effect of gas-containing microspheres and echo contrast agents on free radical formation by ultrasound. Free Radic Biol Med 1998;25:605–612.
Edmonds PD, Sancier KM. Evidence for free radical production by ultrasonic cavitation in biological media. Ultrasound Med Biol 1983;9:635–639.
Unger EC, Porter T, Culp W, et al. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev 2004;56:1291–1314.
Lum AF, Borden MA, Dayton PA, et al. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release 2006;111:128–134.
Suzuki R, Takizawa T, Negishi Y, et al. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release 2007;117:130–136.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Okada, K., Kudo, N., Kondo, T. et al. Contributions of mechanical and sonochemical effects to cell membrane damage induced by single-shot pulsed ultrasound with adjacent microbubbles. J Med Ultrasonics 35, 169–176 (2008). https://doi.org/10.1007/s10396-008-0192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-008-0192-0