Abstract
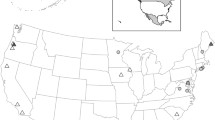
Amphibians face an extinction crisis with no precedence. Two emerging infectious diseases, ranaviral disease caused by viruses within the genus Ranavirus and chytridiomycosis due to Batrachochytrium dendrobatidis (Bd), have been linked with amphibian mass mortalities and population declines in many regions of the globe. The African clawed frog (Xenopus laevis) has been indicated as a vector for the spread of these pathogens. Since the 1970s, this species has been invasive in central Chile. We collected X. laevis and dead native amphibians in Chile between 2011 and 2013. We conducted post-mortem examinations and molecular tests for Ranavirus and Bd. Eight of 187 individuals (4.3 %) tested positive for Ranavirus: seven X. laevis and a giant Chilean frog (Calyptocephallela gayi). All positive cases were from the original area of X. laevis invasion. Bd was found to be more prevalent (14.4 %) and widespread than Ranavirus, and all X. laevis Bd-positive animals presented low to moderate levels of infection. Sequencing of a partial Ranavirus gene revealed 100 % sequence identity with Frog Virus 3. This is the first report of Ranavirus in Chile, and these preliminary results are consistent with a role for X. laevis as an infection reservoir for both Ranavirus and Bd.

Similar content being viewed by others
References
Balseiro A, Dalton KP, Del Cerro A, Márquez I, Parra F, Prieto JM, Casais R (2010) Outbreak of common midwife toad virus in alpine newts (Mesotriton alpestris cyreni) and common midwife toads (Alytes obstetricans) in Northern Spain: A comparative pathological study of an emerging ranavirus. The Veterinary Journal 186:256-258
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and central America. Proceedings of the National Academy of Sciences of the United States of America 95:9031-9036
Bourke J, Ohst T, Graser Y, Bohme W, Plotner J (2011) New records of Batrachochytrium dendrobatidis in Chilean frogs. Diseases of Aquatic Organisms 95:259-261
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms 60:141-148
Cunningham AA, Langton TES, Bennett PM, Lewin JF, Drury SEN, Gough RE, MacGregor SK (1996) Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 351:1539-1557
Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R (1999) Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases 5:735-748
De Jesús Andino F, Chen G, Li Z, Grayfer L, Robert J (2012) Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 432:435-443
Duffus ALJ, Waltzek TB, Stöhr AC, Allender MC, Gotesman M, Whittington RJ, Hick P, Hines MK, Marschang RE (2015) Distribution and host range of ranaviruses. In: Ranaviruses: lethal pathogens of ectothermic vertebrates, Gray MJ, Chinchar VG (editors), New York, Springer, pp 71-104
Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, Clare F, Bosch J, Cunningham AA, Weldon C, du Preez LH, Anderson L, Pond SLK, Shahar-Golan R, Henk DA, Fisher MC (2011) Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proceedings of the National Academy of Sciences of the United States of America 108:18732-18736
Fisher MC, Garner TWJ (2007) The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21: 2-9
Fisher MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63:291-310
Fox SF, Greer AL, Torres-Cervantes R, Collins JP (2006) First case of ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Diseases of Aquatic Organisms 72:87-92
Galli L, Pereira A, Marquez A, Mazzoni R (2006) Ranavirus detection by PCR in cultured tadpoles (Rana catesbeiana Shaw 1802) from South America. Aquaculture 257:78-82
Garland S, Baker A, Phillott AD, Skerratt LF (2010) BSA reduces inhibition in a TaqMan assay for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 92:113-116
Gower DJ, Doherty-Bone T, Loader SP, Wilkinson M, Kouete MT, Tapley B, Orton F, Daniel OZ, Wynne F, Flach E, Müller H, Menegon M, Stephen I, Browne RK, Fisher MC, Cunningham AA, Garner TWJ (2013) EcoHealth 10:173-183
Green DE, Converse KA, Schrader AK (2002) Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Annals of the New York Academy of Sciences 969:323-339
Greenspan SE, Calhoun AJK, Longcore JE, Levy MG (2012) Transmission of Batrachochytrium dendrobatidis to wood frogs (Lithobates sylvaticus) via a bullfrog (L. catesbeianus) vector. Journal of Wildlife Diseases 48:575-582
Greer AL, Berrill M, Wilson PJ (2005) Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Diseases of Aquatic Organisms 67:9-14
Hanselmann R, Rodriguez A, Lampo M, Fajardo-Ramos L, Aguirre AA, Kilpatrick AM, Rodriguez JP, Daszak P (2004) Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biological Conservation 120:115-119
Holopainen R, Ohlemeyer S, Schütze H, Bergmann SM, Tapiovaara H (2009) Ranavirus phylogeny and differentiation based on major capsid protein, DNA polymerase and neurofilament triplet H1-like protein genes. Diseases of Aquatic Organisms 8:81-91
Hudson MA, Young RP, Lopez J, Martin L, Fenton C, McCrea R, Griffiths RA, Adams S-L, Gray G, Garcia G, Cunningham AA (2016) In-situ itraconazole treatment improves survival rate during an amphibian chytridiomycosis epidemic. Biological Conservation 195:37-45
Hyatt AD, Gould AR, Zupanovic Z, Cunningham AA, Hengstberger S, Whittington RJ, Kattenbelt J, Coupar BE (2000) Comparative studies of piscine and amphibian iridoviruses. Archives of Virology 145:301-331
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 73:175-192
James TY, Toledo LF, Rödder D, Leite DS, Belasen A, Betancourt-Román CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV, Ruggeri J, Collins JP, Burrowes P, Lips KR, Zamudio KR, Longcore JE (2015) Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecology and Evolution 5:4079-4097
Jancovich JK, Davidson EW, Morado JF, Jacobs BL, Collins JP (1997) Isolation of a lethal virus from the endangered tiger salamander Ambystoma tigrinum stebbinsi. Diseases of Aquatic Organisms 31:161-167
Jancovich JK, Steckler NK, Waltzek TB (2015) Ranavirus taxonomy and phylogeny In: Ranaviruses: lethal pathogens of ectothermic vertebrates, Gray MJ, Chinchar VG (editors), New York, Springer, pp 59-70
Kik M, Martel A, Spitzen-van der Sluijs A, Pasmans F, Wohlsein P, Gröne A, Rijks JM (2011) Ranavirus-associated mass mortality in wild amphibians, The Netherlands, 2010: A first report. Veterinary Journal 190:284-286
Liu X, Rohr JR, Li YM (2013) Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 280:20122506
Lobos G, Jaksic FM (2005) The ongoing invasion of African clawed frogs (Xenopus leavis) in Chile: causes of concern. Biodiversity and Conservation 14:429-439
Mao J, Hedrick RP, Chinchar VG (1997) Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 229:212-220
Mazzoni R, de Mesquita AJ, Fleury LF, de Brito WM, Nunes IA, Robert J, Morales H, Coelho AS, Barthasson DL, Galli L, Catroxo MH (2009) Mass mortality associated with a Frog Virus 3-like ranavirus infection in farmed tadpoles Rana catesbeiana from Brazil. Diseases of Aquatic Organisms 86:181-191
Mazzoni, R., Cunningham, A. A., Daszak, P., Apolo, A., Perdomo, E., and Speranza, G. (2003) Emerging pathogen of wild amphibians in frogs (Rana catesbeiana) farmed for international trade. Emerging Infectious Diseases 9:995-998
Miller DL, Gray MJ, Storfer A (2011) Ecopathology of ranaviruses infecting amphibians. Viruses 3:2351-2373
Murray KA, Retallick RWR, Puschendorf R, Skerratt LF, Rosauer D, McCallum HI, Berger L, Speare R, VanDerWal J (2011) Assessing spatial patterns of disease risk to biodiversity: implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. Journal of Applied Ecology 4:163-173
Muths E, Gallant AL, Campbell EHC, Battaglin WA, Green DE, Staiger JS, Walls SC, Gunzburger MS, Kearney RF (2006) The amphibian research and monitoring initiative (ARMI): 5-year report. US geological survey scientific investigations report 2006-5224
North AC, Hodgson DJ, Price SJ, Griffiths AGF (2015) Anthropogenic and ecological drivers of amphibian disease (ranavirosis). PLoS ONE 10:e0127037
OIE (World Organisation for Animal Health) (2015) Manual of diagnostic tests for aquatic animals 2015. www.oie.int/international-standard-setting/aquatic-manual/access-online/. Accessed March 14, 2016
Olson DH, Ronnenberg KL (2014) Global Bd mapping project: 2014 update. FrogLog 22:17-21
Price S, Garner TWJ, Nichols RA, Balloux F, Ayres C, Mora-Cabello de Alba A, Bosch J (2014) Collapse of amphibian communities due to and introduced Ranavirus. Current Biology 24:2586-2591
Phillot AD, Speare R, Hines HB, Skerrat LF, Meyer E, McDonald KR, Cashins SD, Mendez D, Berger L (2010) Minimising exposure of amphibians to pathogens during field studies. Diseases of Aquatic Organisms 92:175-185
Pounds AJ, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, la Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sanchez-Azofeifa GA, Still CJ, Young BE (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161-167
Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infection and Immunity 78:3981-3992
Robert J, Abramowitz L, Gantress J, Morales HD (2007) Xenopus laevis: a possible vector of ranavirus infection? Journal of Wildlife Diseases 43:645-652
Rohr JR, Halstead NT, Raffel TR (2011) Modelling the future distribution of the amphibian chytrid fungus: the influence of climate and human-associated factors. Journal of Applied Ecology 48:174-176
Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P (2006) The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3:35-40
Schloegel LM, Ferreira CM, James TY, Hipolito M, Longcore JE, Hyatt AD, Yabsley M, Martins AMCRPF, Mazzoni R, Davies AJ, Daszak P (2010) The North American bullfrog as a reservoir for the spread of Batrachochytrium dendrobatidis in Brazil. Animal Conservation 13:53-61
Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, Lee M, Zhao S, Wangen C, Ferreira CM, Hipolito M, Davies AJ, Cuomo CA, Daszak P, James TY (2012) Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Molecular Ecology 21:5162-5177
Sleeman J, Brand C, Wright S (2012) Strategies for wildlife disease surveillance. In: New Directions in Conservation Medicine, Aguirre A, Ostfeld R, Daszak P (editors), New York, Oxford University Press, pp 539-551
Smith KF, Acevedo-Whitehouse K, Pedersen AB (2009) The role of infectious diseases in biological conservation. Animal Conservation 12:1-12
Solís R, Lobos G, Walker S, Fisher M, Bosch J (2010) Presence of Batrachochytrium dendrobatidis in feral populations of Xenopus laevis in Chile. Biological Invasions 12:1641-1646
Solís R, Penna M, De la Riva I, Fisher MC, Bosch J (2015) Presence of Batrachochytrium dendrobatidis in anurans from the Andes highlands of northern Chile. Herpetological Journal 24:55-59
Soto-Azat C, Valenzuela-Sánchez A, Clarke BT, Busse K, Ortiz JC, Barrientos C, Cunningham AA (2013a) Is chytridiomycosis driving Darwin’s frogs to extinction? PLoS ONE 8:e79862
Soto-Azat C, Valenzuela-Sánchez A, Collen B, Rowcliffe MC, Veloso A, Cunningham AA (2013b) The population decline and extinction of Darwin’s frogs. PLoS ONE 8:e66957
Soto-Azat C, Valenzuela-Sánchez A, Ortiz JC, Díaz-Páez H, Castro C, Charrier A, Correa C, Cuevas C, Lobos G, Mendez MA, Penna M, Peñafiel-Ricaurte A, Rabanal F, Vélez-R CM, Vidal MA, Angulo A (2015) ASG Chile leads update of the extinction risk of Chilean amphibians for the IUCN red list of threatened species. FrogLog 23:6-7
St-Amour V, Wong WM, Garner TWJ, Lesbarreres D (2008) Anthropogenic influence on prevalence of 2 amphibian pathogens. Emerging Infectious Diseases 14:1175-1176
Stöhr AC, Hoffmann A, Papp T, Robert N, Pruvost NBM, Reyer HU, Marschang RE (2013) Long-term study of an infection with ranaviruses in a group of edible frogs (Pelophylax kl. esculentus) and partial characterization of two viruses based on four genomic regions. Veterinary Journal 197:238-244
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783-1786
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30:2725-2729
Teacher AGF, Cunningham AA, Garner TWJ (2010) Assessing the long-term impact of Ranavirus infection in wild common frog populations. Animal Conservation 13:514-522
Tinsley RC, Loumont C, KOBEL HR (1996) Geographical distribution and ecology. In: The biology of Xenopus, Tinsley RC, Kobel HR (editors), Oxford, Clarendon Press, pp 35-39
Une Y, Sakuma A, Matsueda H et al (2009) Ranavirus outbreak in North American bullfrogs (Rana catesbeiana), Japan. Emerging Infectious Diseases 15:1146-1147
Veloso A, Formas R, Gerson H (2010) Calyptocephalella gayi. The IUCN red list of threatened species 2010: e.T4055A10332590. http://dx.doi.org/10.2305/IUCN.UK.2010-2.RLTS.T4055A10332590.en. Accessed January 11, 2016
Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerrat LF, Speare R (2009) Pathogenesis of amphibian chytridiomycosis, a cause of catastrophic amphibian declines. Science 326:582-585
Warne RW, LaBumbard B, LaGrange S, Vredenburg VT, Catenazzi A (2016) Co-infection by chytrid fungus and ranaviruses in wild and harvested frogs in the tropical Andes. PLoS ONE 11:e0145864
Webb R, Berger L, Mendez D, Speare R (2005) MS-222 (tricaine methane sulfonate) does not kill the amphibian chytrid fungus Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 68:89-90
Zupanovic Z, Lopez G, Hyatt AD, Green B, Bartran G, Parkes H, Whittington RJ, Speare R (1998) Giant toads Bufo marinus in Australia and Venezuela have antibodies against ‘ranaviruses’. Diseases of Aquatic Organisms 32:1-8
Acknowledgments
This research was funded by the Zoological Society of London (ZSL) EDGE Fellowship Programme; the Dirección General de Investigación y Doctorado, Universidad Andrés Bello (DI-526-14/R), NERC standard Grant NE/M000338/1, NE/M00080X/1, NE/M000591/1, and the Chilean National Science and Technology Fund (FONDECYT iniciación N° 11140902).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Collection of wild amphibians was approved under the permits of the Chilean Agriculture and Livestock Service (Nº7993/2010, 300/2012, 5666/2013) and the Universidad Andres Bello Bioethical Committee (1939/2012 and 19/2013), and followed the Bioethic Guidelines of the Comisión Nacional de Investigación Científica y Tecnologica de Chile (CONICYT 2009).
Rights and permissions
About this article
Cite this article
Soto-Azat, C., Peñafiel-Ricaurte, A., Price, S.J. et al. Xenopus laevis and Emerging Amphibian Pathogens in Chile. EcoHealth 13, 775–783 (2016). https://doi.org/10.1007/s10393-016-1186-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-016-1186-9