Abstract
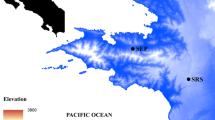
The pathogenic chytrid fungus, Batrachochytrium dendrobatidis, has been implicated as the main driver of many enigmatic amphibian declines in neotropical sites at high elevation. Batrachochytrium dendrobatidis is thought to be a waterborne pathogen limited by temperature, and the extent to which it persists and causes disease in amphibians at lower elevations in the neotropics is not known. It also is unclear by what mechanism(s) B. dendrobatidis has emerged as a pathogenic organism. To test whether B. dendrobatidis is limited by elevation in Panamá, we sought to determine the prevalence and intensity of B. dendrobatidis in relation to anuran abundance and diversity using quantitative PCR (qPCR) analyses. Sites were situated at varying elevations, from 45 to 1215 m, and were at varying stages of epizootic amphibian decline, including pre-epizootic, mid-epizootic, 2 years post-epizootic, and 10 years post-epizootic. Batrachochytrium dendrobatidis was found in all sites regardless of elevation or stage of epizootic decline. Levels of prevalence and infection intensity were comparable across all sites except at the mid-epizootic site, where both prevalence and intensity were significantly higher than at other sites. Symptoms of chytridiomycosis and corresponding declines in amphibian populations were variably seen at all elevations along a post-epizootic gradient. Because it is inherently difficult to prove a negative proposition, it can neither be proven that B. dendrobatidis is truly not present where it is not detected nor proven that it is only recently arrived where it is detected. Thus, there will always be doubts about whether B. dendrobatidis is enzootic or invasive. In any case, our results, coupled with current knowledge, suggest most clearly that the disease, chytridiomycosis, may be novel and invasive, and that the pathogen, B. dendrobatidis either is, or is becoming, globally ubiquitous.




Similar content being viewed by others
References
Alford RA, Bradfield KS, Richards SJ (2007) Global warming and amphibian losses. Nature 447:E3–E4.
Amiche M, Aurelia AS, Thierry NP, Nicholas P (1999) The dermaceptin precursors: a protein family with a common preproregion and a variable C-terminal antimicrobial domain. FEBS Letters 456:352–356.
Annis SL, Dastoor FP, Ziel H, Daszak P, Longcore JE (2004) A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. Journal of Wildlife Diseases 40:420–428.
Aplin K, Kirkpatrick P (2000) Chytridiomycosis in southwest Australia: historical sampling documents the date of introduction, rates of spread and seasonal epidemiology, and sheds new light on chytrid ecology. In: Getting the Jump! On Amphibian Disease, Conference and Workshop Compendium. Williams K, Speare R (editors), Cairns: Rainforest CRC, pp 24.
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences USA 95:9031–9036.
Berger L, Speare R, Hines HB, Marantelli G, Hyatt AD, McDonald KR, Skerratt LF, Olsen V, Clarke JM, Gillespie G, Mahony M, Sheppard N, Williams C, Tyler MJ (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal 82:434–439.
Bosch J, Martinez-Solano I, Garcia-Paris M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biological Conservation 97:331–337.
Bosch J, Carrascal LM, Duran L, Walker S, Fisher MC (2007) Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proceedings of the Royal Society B 274:253–260.
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR Assay. Diseases of Aquatic Organisms 60:141–148.
Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ (2005) Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians. Ecology 86:3149–3159.
Campbell JA (1999) Distribution patterns of amphibians in Middle America. In: Patterns of Distribution of Amphibians: A Global Perspective, Duellman WA (editor), Baltimore: Johns Hopkins University Press, pp 111–210.
Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Developmental and Comparative Immunology 23:459–472.
Carey C, Bruzgul JE, Livo LJ, Walling ML, Kuehl KA, Dixon BF, Pessier AP, Alford RA, Rogers KB (2006) Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3:5–21.
Clem LS, Miller NW, Bly JE (1991) Evolution of lymphocyte subpopulations, their interactions, and temperature sensitivities. In: Phylogenesis of Immune Functions, Warr GW Cohen N (editors), Boca Raton, FL: CRC Press, pp 191–210.
Colwell RK (2009) EstimateS: Statistical estimation of species richness and shared species from samples. Version 8.2. User’s Guide and application. http://purl.oclc.org/estimates
Crawley MJ (2007) The R Book. Chichester: Wiley.
Daszak P, Strieby A, Cunningham AA, Longcore J, Brown CC, Porter D (2004) Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetological Journal 14:201–207.
Devillers J, Extrayat JM (editors) (1992) Ecotoxicity of Chemicals to Amphibians, Philadelphia: Gordon and Breach.
DiGiacomo RF, Koepsell TD (1986) Sampling for detection of infection or disease in animal populations. Journal of the American Veterinary Medicine Association 189:22–23.
Fisher, MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63:291–310.
Garner TWJ, Walker S, Bosch J, Hyatt AD, Cunningham AA, Fisher MC (2005) Chytrid fungus in Europe. Emerging Infectious Diseases 11:1639–1641.
Hanselmann R, Rodriguez A, Lampo M, Fajardo-Ramos L, Aguirre AA, Kilpatrick AM, Rodriguez JP, Daszak P (2004) Presence of an emerging pathogen of amphibians in introduced bullfrogs Rana catesbeiana in Venezuela. Biological Conservation 120:115–119.
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 73:175–192.
James TY, Litvintseva AP, Vilgalys R, Morgan JAT, Taylor JW, Fisher MC, Berger L, Weldon C, du Preez L, Longcore JE (2009) Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathogens 5 e1000458:1–12.
Johnson ML, Speare R (2003) Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerging Infectious Diseases 9:922–925.
Kriger KM, Hero JM (2007) Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. Journal of Zoology 271:352–359.
Kriger KM, Hero JM (2008) Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral Ecology 33:1022–1033.
Kriger KM, Hero JM (2009) Chytridiomycosis, amphibian extinctions, and lessons for the prevention of future panzootics. Ecohealth 6:6–10.
Kriger KM, Hines HB, Hyatt AD, Boyle DG, Hero J-M (2006a) Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Diseases of Aquatic Organisms 71:141-148.
Kriger KM, Hero J-M, Ashton KJ (2006b) Cost efficiency in the detection of chytridiomycosis using PCR assay. Diseases of Aquatic Organisms 71:149–154.
Kriger KM, Ashton KJ, Hines HB, Hero J-M (2007) On the biological relevance of a single Batrachochytrium dendrobatidis zoospore: a reply to Smith (2007). Diseases of Aquatic Organisms 73:257–260.
La Marca E, Lips KR, Lotters S, Puschendorf R, Ibáñez R, Rueda-Almonacid JV, Schulte R, Marty C, Castro F, Manzanilla-Puppo J, Garcia-Perez JE, Bolanos F, Chaves G, Pounds AJ, Toral E, Young BE (2005) Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37:190–201.
Lips KR, Reeve JD, Witters LR (2003) Ecological traits predicting amphibian population declines in Central America. Conservation Biology 17:1078–1088.
Lips KR, Burrowes PA, Mendelson JR, Parra-Olea G (2005a) Amphibian population declines in Latin America: a synthesis. Biotropica 37:222–226.
Lips KR, Burrowes PA, Mendelson JR, Parra-Olea G (2005b) Amphibian declines in Latin America: widespread population declines, extinctions, and impacts. Biotropica 37:163–165.
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proceedings of the National Academy of Sciences of USA 103:3165–3170.
Lips KR, Diffendorfer J, Mendelson III JR, Sears MW (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. Plos Biology 6:441–454.
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227.
McDonald KR, Mendez D, Muller R, Freeman AB, Speare R (2005) Decline in the prevalence of chytridiomycosis in frog populations in North Queensland, Australia. Pacific Conservation Biology 11:114–120.
Morgan JAT, Vredenburg VT, Rachwicz LJ, Knapp RA, Stice MJ, Tunstall T, Bingham RE, Parker JM, Longcore JE, Moritz C, Briggs CJ, Taylor JW (2007) Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences of USA 104:13845–13850.
Oliveira de Queiroz Carnaval AC, Puschendorf R, Peixoto OL, Verdade VK, Rodrigues MT (2006) Amphibian chytrid fungus broadly distributed in the Brazilian Atlantic rainforest. Ecohealth 3:41–48.
Pounds JA, Crump ML (1994) Amphibian declines and climate disturbances: the case of the golden toad and the harlequin frog. Conservation Biology 8:72–85.
Pounds JA, Fogden MPL, Campbell JH (1999) Biological response to climate change on a tropical mountain. Nature 398:611–615.
Pounds JA, Bustamante MA, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca EL, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sanchez-Azofeifa GA, Still CJ, Young BE (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167.
Puschendorf R, Bolaños F, Chaves G (2006) The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biological Conservation 132:136–142.
R Development Core Team (2008) R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org
Rachowicz LJ, Hero J-M, Alford RA, Taylor JW, Morgan JAT, Vredenburg VT, Collins JP, Briggs CJ (2005) The novel and endemic pathogen hypothesis: competing explanations for the origin of emerging infectious diseases of wildlife. Conservation Biology 19:1441–1448.
Retallick RWR, McCallum H, Speare R (2004) Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biology 2:1965–1071.
Ribas L, Li MS, Doddington BJ, Robert J, Seidel JA, Kroll JS, Zimmerman LB, Grassly NC, Garner TWG, Fisher MC (2009) Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PloS ONE 4:1–10.
Rollins-Smith LA, Reinert LK, O’Leary CJ, Houston LE, Woodhams DC (2005) Antimicrobial peptide defenses in amphibian skin. Integrative and Comparative Biology 45:137–142.
Ron SR, Duellman WE, Coloma LA, Bustamante MR (2003) Population declines of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. Journal of Herpetology 37:116–126.
Savage JM (2002) The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas, Chicago: The University of Chicago Press.
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillot AD, Hines HB, Kenyon N (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4:125–137.
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786.
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large scale amphibian population extinction. Proceedings of the National Academy of Sciences of the USA 107:9689–9694.
Walker SF, Bosch J, Gomez V, Garner TWJ, Cunningham AA, Schmeller DS, Ninyerola M, Henk DA, Ginestet C, Arthur C-P, Fisher MC (2010) Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecology Letters 13:372–382.
Waller DM, O’Malley DM, Gawler SC (1987) Genetic variation in the extreme enzootic Pedicularis furbishiae (Scrophulariaceae). Conservation Biology 1:335–340.
Woodhams DC, Alford RA (2005) Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conservation Biology 19:1449–1459.
Woodhams DC, Voyles J, Lips KR, Carey C, Rollins-Smith LA (2006) Predicted disease susceptibility in a Panamanian amphibian assemblage based on skin peptide defenses. Journal of Wildlife Diseases 42:207–218.
Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA (2008a) Life history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology 89:1627–1639.
Woodhams, DC, Kilburn VL, Reinert LK, Voyles J, Medina D, Ibáñez RD, Hyatt AD, Boyle DG, Pask JD, Green DM, Rollins-Smith LA (2008b) Chytridiomycosis and amphibian population declines continue to spread eastward in Panamá. EcoHealth 5:268–274.
Woodhams D, Kenyon N, Bell S, Alford R, Chen S, Billheimer D, Shyr Y, Rollins-Smith L (2010) Adaptations of skin peptide defences and possible response to the amphibian chytrid fungus in populations of Australian green-eyed tree frogs, Litoria genimaculata. Diversity and Distributions 16:703–712.
Wright K, Berger L, Nichols DK, Speare R, Sredl MJ, Pessier A, Johnson B (2001) Roundtable: amphibian population decline. Journal of Herpetological Medicine and Surgery 11:14–27.
Acknowledgments
We thank Julie Ray, Gisela Reina, Andrew Crawford, Vicky Flechas, Kate and Dave Turner, Sky Oestreicher, Erin Trimble, Dustin Raab, Peter McGaw, Amanda Kilburn, César and Fidel Jaramillo, and Frank Solís for help in the field and Anne Bramard (Genome Quebec) and Catherine Brisson for help with the molecular analyses. This research was supported by an NSERC CGS M grant to VLK and an NSERC Canada Discovery Grant to DMG.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Kilburn, V.L., Ibáñez, R., Sanjur, O. et al. Ubiquity of the Pathogenic Chytrid Fungus, Batrachochytrium dendrobatidis, in Anuran Communities in Panamá. EcoHealth 7, 537–548 (2010). https://doi.org/10.1007/s10393-010-0634-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-010-0634-1