Abstract
The Tasmanian devil, Sarcophilus harrisii, is the largest extant marsupial carnivore. In 1996, a debilitating facial tumor was reported. It is now clear that this is an invariably lethal infectious cancer. The disease has now spread across the majority of the range of the species and is likely to occur across the entire range within 5 to 10 years. The disease has lead to continuing declines of up to 90% and virtual disappearance of older age classes. Mark-recapture analysis and a preliminary epidemiological model developed for the population with the best longitudinal data both project local extinction in that area over a timeframe of 10 to 15 years from disease emergence. However, the prediction of extinction from the model is sensitive to the estimate of the latent period, which is poorly known. As transmission appears to occur by biting, much of which happens during sexual encounters, the dynamics of the disease may be typical of sexually transmitted diseases. This means that transmission is likely to be frequency-dependent with no threshold density for disease maintenance. Extinction over the entire current range of the devil is therefore a real possibility and an unacceptable risk.
Similar content being viewed by others
INTRODUCTION
Signs characteristic of Tasmanian devil facial tumor disease (DFTD) were first detected in photographs taken at Mt. William National Park, Northeast Tasmania in 1996. The first case of DFTD confirmed through histological examination was collected in 1997 (Loh et al., 2006) near the photographic report. It has now spread over the majority of the range of the species, leading to major population declines. In this article, we review and update current knowledge about the distribution and spatial spread of the disease, its observed impact on affected host populations, and models to predict its consequences for host population dynamics.
DENSITY AND DISTRIBUTION OF DEVILS
Prior to the emergence of DFTD, Tasmanian devils were distributed throughout the Tasmanian mainland, though at very low densities in the Southwest (Jones and Rose, 1996). The findings of Hawkins et al. (2006), together with our work until March 2007, continuing the same spotlighting survey and trapping methods, indicate that this distribution has remained unchanged following disease emergence but that densities are now much reduced. Estimates of the overall size of the devil population are difficult to obtain. Mark-recapture estimates from the DFTD program (“standard trapping survey” methods detailed in Hawkins et al., 2006) are in the range of 0.8–2.9 individuals per km2 (three sites in the disease-free Northwest), and 0.3–1.4 individuals per km2 (four sites in the confirmed-disease region) (including a 2-km buffer zone around the trapping grid). These mark-recapture sites were selected as areas known to hold devils, so are likely to be relatively high density. All evidence points to extremely low densities in the wet and mountainous Southwest region (Fig. 1; Jones and Rose, 1996; Hawkins et al., 2006). Given that the total mainland area of Tasmania is about 64,000 km2, these findings indicate that previous informal population estimates for the 1990s of 130,000–150,000 individuals (e.g., quoted by Bradshaw and Brook, 2005) are overestimates. Early published mark-recapture density estimates of at least 5.2 km−2 (Guiler, 1970) are considerably higher than ours, but without detailed information on the spatial configuration of the trapping grid, determining the area effectively sampled is difficult.
Tasmania, showing devil distribution and places mentioned in the text. The World Heritage Area in the Southwest delineates a region of very low devil population density, both before and after DFTD emergence (Jones and Rose, 1996; Hawkins et al., 2006).
It has been suggested that devil population numbers may have fluctuated substantially in the 19th century, perhaps in association with disease (Guiler, 1964, 1992). This idea was used as the basis of a model (Bradshaw and Brook, 2005), suggesting that density-dependent disease may have been responsible for these previous fluctuations. Owens and Pemberton (2005) investigated the anecdotal evidence for substantial fluctuations in devil density, and found no grounds for any firm conclusion to be drawn. Devils have unusually low genetic diversity for a mammal (Jones et al., 2004), which is consistent with a founder effect when Tasmania became isolated at the end of the last Ice Age, but is not necessarily evidence of any more recent bottleneck.
DISTRIBUTION AND SPREAD OF DISEASE
Two methods have been used to estimate the distribution and spread of DFTD across Tasmania. First, roadkill reports and trapping data were mapped with associated dates of first report of confirmed DFTD (details in Hawkins et al., 2006). These data are reliable, but spatially patchy and are likely to underestimate the disease distribution. Second, 132 10-km spotlighting transects have been surveyed annually for a range of wildlife species since 1985 (Hocking and Driessen, 1992; Hawkins et al., 2006). Further transects have been progressively added, so that 177 were available for analysis since the first DFTD report. After aggregating transects into 54 neighboring groups of one to six (accounting for topographical features), sightings of devils were visually assessed for an acute, sustained decline over time (usually over at least 2 years). In 15 of these groups, there was a clear sustained decline, and the year at which this decline commenced was treated as the year of emergence of disease. In a further 11 groups, there was a less dramatic decline; these cases were only used where the apparent year of emergence was compatible with that indicated by nearby confirmed or anecdotal disease reports. Groups that showed no decline were assumed disease-free and arbitrarily given an emergence year of 2007, (i.e., 1 year into the future.) Inevitably, this process is rather subjective. Further, the approach presumes that all the declines were due to disease. Potentially, however, it may produce a finer scale spatial representation of disease spread than can be derived from confirmed-disease reports alone.
The distribution of confirmed DFTD cases in March 2007, based on a minimum convex polygon around all confirmed DFTD locations, constituted 59% of the Tasmanian mainland (Fig. 2). This is an underestimate, because the apparent disease front could not be intensively monitored, especially in the remote Southwest region where devils are at extremely low density. In this region (indicated in Fig. 1), remotely triggered cameras were distributed mostly near the suspected front from 2004 to 2006. During 1130 camera nights, 42 distinguishable individuals were photographed, none of which exhibited DFTD signs (yielding an upper 95% bound on population prevalence of 0.084). However, subsequent to a small scale camera trapping survey, a DFTD case was confirmed in a roadkill at Strathgordon (Fig. 1), in the Southwest, isolated by water. Confidence in disease absence is much higher in the North of this region and West of the apparent disease front: a total of 705 individuals have been trapped there in 2004–2007 with no disease signs found (of which 343 were examined in 2006–2007, yielding an upper bound on population prevalence of 0.01).
To visualize our best understanding of the spread of DFTD across Tasmania, year of emergence based on the spotlighting data was used as a foundation for interpolation through splining. Confirmed reports were then used to validate and refine the disease spread surface (Fig. 2); confirmed reports were only included where they indicated earlier disease emergence than that inferred by spotlighting data. Overall, the two sources of data were consistent. In areas of the Northwest where there were no spotlighting data, trapping data (where at least 50 individuals had been caught and no sign of disease found) were also incorporated in the same way as spotlight data showing no decline.
At a coarse scale, the pattern of spread indicated by Figure 2 supports the hypothesis that this is an infectious disease and that a single origin, rather than multiple origins, is implied. It appears that DFTD has spread at a variable rate. Assuming DFTD emerged shortly before 1996, it has spread approximately 270 km South in 11 years through fairly continuous forested habitat, while during the same period it spread only 190 km West–Southwest though heterogeneous habitat. It appears to have spread down the East coast by as much 155 km in 3 years, from 1996 to 1999. Devil movements can be highly variable: Individuals have been known to travel by as much as 16–25 km in a single direction in one night (Guiler, 1978; Pemberton 1990) [M.E.J., personal observation], while individuals may also be recaptured less than a kilometer from the first trap site, even if several nights have elapsed between captures [Hawkins et al., unpublished data]. The slow rate of disease spatial spread relative to the movement rate of individual devils suggests that R 0 is not very large.
In the Freycinet National Park and surrounds, the region with the best longitudinal data (several trapping trips each year from 1999–2006), a logistic model suggests an accelerating rate of spread as the disease moves Southwards (Likelihood ratio for distance–time interaction 11.78, P = 0.0001), with a mean rate of spread of about 7 km yr−1.
The furthest point from the known front, as indicated by confirmed reports, is the Northwest tip of Tasmania, at a distance of approximately 140 km. Given that the spread rate is variable, between 7 km yr−1 down the Freycinet peninsula to 52 km yr−1 down Tasmania’s East coast, DFTD could take 3–20 years to reach a statewide distribution, with disease likely to reach the Northwest within 3–10 years.
IMPACT OF DISEASE
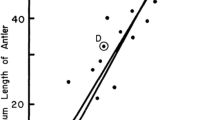
Mark-recapture data from before and after disease arrival are available from two sites on the East coast of Tasmania, Mt. William, and the Freycinet Peninsula (see Fig. 1). Population size on a trapping grid at Mt. William was estimated at over 200 individuals in 1984–1985 (Pemberton 1990), but had declined to about 50 individuals when a larger 28 km2 trapping grid encompassing Pemberton's grid was surveyed in August 2004 (Hawkins et al., 2006), with a further decline to 25 by July 2006 [Lachish et al., 2007]. The estimated population size at Freycinet following disease arrival (Lachish et al. 2007) also showed a major (and continuing) decline of at least 60% (Fig. 3). More extensive, but less precise evidence of declines comes from spotlighting data (Hawkins et al., 2006). Recent updating of these data shows that devil sightings at 167 annually surveyed 10-km routes across mainland Tasmania declined by 53% from 1992-1995 to 2003-2006. Sightings from 25 routes in Northeast Tasmania (where DFTD appears to have first emerged), declined by 89% over the same period—a similar decline rate as indicated by the mark-recapture data for Mt. William, which lies in this region.
Figure 4 shows the mean number of sightings per 10 km of the spotlight transects, aggregated into five regions. This is a much coarser level of aggregation than was used to derive the interpolated disease arrival in Figure 2. The disease was first recorded from the Northeast region, as described above. Here, a steady decline from the mid-1990s is obvious. As shown in Figure 2, the disease emerged in the East of Tasmania in the late 1990s. There is a similar pronounced decline in this region, although it commenced a little later. This region is bounded by the Tamar estuary in the North (which is prominent on Fig. 2) and the Forestier Peninsula in the South (see Fig. 1 for location). In the Northwest, which the disease has not reached, sightings have been high, but variable, and show no evidence of sustained decline to this point. The Southwest has a low devil density and disease has been detected only in the last year. As yet, there is no evidence of decline. The remainder of the transects have been grouped into the “Midlands” panel. First confirmed records of the disease in this area (see Fig. 2) occurred in 2003, but it did not become widespread until 2004–2005. Here, there is some evidence of a recent decline commencing in about 2003, but clearly more years of data are required to confirm this.
Estimated annual survival of adults first marked as adults (i.e., first caught at age 2+) has declined dramatically at Freycinet from approximately 0.5 before disease arrival to very close to zero. Annual survival of adults first marked at age 1+ has also declined, but not as rapidly (Lachish et al., 2007). The slower decline in survival of animals first marked as 1+ individuals is probably because individuals marked as 1+ are, on average, younger than those first captured as 2+. In the early stages of any epidemic, when force of infection is relatively low, the average age at which disease is acquired is greater than later in the epidemic (Grenfell and Anderson, 1985). There is some empirical evidence [S.L., unpublished; C.H., unpublished] that the disease first occurs in older animals. The very low survival of adults means that females are likely to survive for, at most, one breeding season, with severe consequences for population persistence. Most females do not commence breeding until 2+, although there appears to be increased breeding of 1+ females in diseased populations [M.E.J., S.L., C.H., unpublished data]. DFTD is also associated with a marked change in age structure: Representation of older age classes continues to decrease with time since emergence, even at Mt. William, with no animals older than 3 years being trapped from many affected areas [Jones et al., in preparation; Hawkins et al., in preparation].
WILL DISEASE LEAD TO EXTINCTION?
Two forms of analysis of the Freycinet data project possible local extinction. First, a reverse-time CMR method (Nichols and Hines, 2002; Pradel, 1996) was used to estimate the finite rate of change λ of the adult population. Prior to disease arrival, the population appears to have been stable (confidence intervals for λ over the first 2 years include 1.0), with an immediate decline in growth rate following the onset of disease. The final estimate for λ suggests that the adult segment of the population declined immediately following disease arrival and is now approximately halving annually (Lachish et al., 2007). Given that the adult (2+) population size in the Freycinet study area is estimated to be less than 10 individuals and that the total population is estimated to be less than 50 (Lachish et al., 2007), this estimate of λ projects extinction within a few years.
Second, a preliminary non-spatial age- and sex-structured model was constructed, with discrete yearly age classes for 0–6-year-old animals, and simulated in discrete monthly time steps. Within each age/sex class, animals were split into three discrete disease-related subclasses—Susceptible, Exposed, and Infectious.
The model was initially constructed in the absence of disease, with population regulation occurring via density-dependent effects on juvenile survival and the proportion of females breeding in each age class (both increasing exponentially with decreasing population size). Maximum juvenile survival was set equal to that of 2–4-year-old adults, with density dependence scaled to give a carrying capacity of 1000 animals. Density dependence on the proportion of females breeding was scaled such that the proportions of 1, 2, 3, and 4–6-year-old animals breeding at carrying capacity matched observed proportions in the field [Hawkins et al., unpublished data]. All other parameters were estimated from available data.
DFTD was modeled as a contact-transmitted disease, with transmission based on probabilities of each individual biting each other individual in the population per month. Three potential modes of transmission were identified from bite-wound surveillance data (Hamede et al., 2007)—background transmission, male/male pre-mating transmission, and male/female mating transmission. Background transmission was assumed to occur all year round, with the per-capita rate being the same for both sexes and all ages. The additional per-capita probability of both male/male transmission during the pre-mating period, and male/female transmission during the mating period, was estimated from the bite wound data as twice the background probability. The only impact of disease modeled was disease-induced mortality of infectious individuals.
There is insufficient knowledge of how transmission scales with devil density. Three different transmission functions were trialed: per-capita probability of disease transmission remaining constant, and transmission increasing with decreasing density in either a linear or exponential fashion. Such increases can potentially occur when interactions among individuals are maintained at low density due to behavioral and social reasons, such as co-feeding, antagonistic behavior, and mating. For each transmission function, parameters giving the best simulated fit to the data were obtained using numerical optimization of the simulation model.
Neither the constant nor linear transmission functions allowed a reasonable fit to the available data, while the best-fitting model based on the exponential transmission function gave the fit illustrated in Figure 5. Simulations with this transmission function also adequately replicated observed population declines and changes in population structure.
Predicted (line) and observed (circles) prevalence of DFTD in Tasmanian devils at Freycinet National Park from July 2001 to July 2006 (with July 2001 being the date when the disease was first detected at this site). Predicted values were generated by the best fit simulation model using an exponential transmission function (see text).
The population trajectory simulated by the best-fit model for 2001–2006 is illustrated in Figure 6, as is the predicted population trajectory for 2006–2011. These trajectories are based on a latent period of 12 months (an animal was observed to develop facial tumors after 10 months in captivity). Recent evidence from captive animals suggests that a value of 6 months may be more realistic [Pyecroft, personal communication]. A shorter latent period also fits more closely with field observations of juveniles as young as 13 months of age developing tumors [Hawkins et al., in preparation], given that vertical disease transmission is not known to occur and juveniles do not leave the pouch until approximately 8 months of age. Altering this value has important implications for the predicted impact of disease on the Freycinet Peninsula devil population. Rather than a decline to extinction being predicted by the best-fit model, as was the case with the longer latent period, the best-fit model based on the shorter latent period alters the prediction to a decline leading to a stable population regulated at approximately 15% of its carrying capacity with disease persisting at approximately 7% prevalence.
Predicted decline in the Freycinet National Park Tasmanian devil population, due to the impact of DFTD, from July 2001 to July 2011 (with July 2001 being the date when the disease was first detected at this site). Values were generated by the best fit simulation model using an exponential transmission function (see text).
Irrespective of the predicted outcome of the disease-induced decline, the simulation approach utilized here indicates an ongoing rapid decline of the Freycinet population, in agreement with that inferred from the mark-recapture analysis. Determining whether local extinction is likely to be the ultimate outcome requires more field data. In particular, field confirmation that the exponential transmission model is valid is required (other transmission models are less likely to lead to predictions of extinction), as is more detail on the latent period of infection in the wild.
DISCUSSION
Conventional epidemiological theory suggests that a pathogen with only a single host is unlikely to drive that host to extinction (de Castro and Bolker, 2005; McCallum and Dobson, 1995). Once the disease has sufficiently reduced host population density, the disease will be unable to transmit effectively and should become extinct before the host. Indeed, in almost all of the cases in the literature in which a disease has likely been responsible for extinction or near extinction, one or more reservoir species, within which the pathogen has a limited effect on survival, exist. Reservoirs result in a strong force of infection for the endangered species occurring even as its numbers decline towards extinction (de Castro et al., 2005; Gog et al., 2002; McCallum and Dobson, 2002). However, this conventional wisdom relies on transmission occurring in a density-dependent rather than a frequency-dependent fashion. If transmission depends on the frequency of infected hosts in the population, rather than their density, there is no threshold population size for disease maintenance and pathogen-induced extinction is a possibility even in the absence of reservoir hosts (de Castro et al., 2005).
If transmission of DFTD occurs primarily from biting and such biting occurs mainly during sexual encounters, then DFTD will possess many of the characteristics of a sexually transmitted disease, one of which is the likelihood of frequency-dependent transmission. Unfortunately, determining the functional form of the transmission function is extremely difficult from empirical data (McCallum et al., 2001). However, there are indications from the monitoring reported in this article, and from the contact rate data reported elsewhere in this issue, that transmission may depend weakly, if at all, on devil population density. For example, DFTD has been confirmed in Strathgordon in the low density Southwest region, and continues to be present in Mt. William even after a 90% population decline. If this is the case, then the extrapolation of current rates of decline to produce a prognosis of extinction on the Freycinet Peninsula in less than 10 years may be valid. If trends in DFTD spread and population decline continue, there is a strong possibility of extinction of the Tasmanian devil due to the disease over a timeframe of perhaps 25–35 years.
The simulation model suggests that whether the disease will lead to extinction is strongly dependent on the length of the latent period. Indeed, such time delays in disease dynamics do commonly act to destabilize host populations (May and Anderson, 1978). Hence, estimation of this critical parameter is clearly a major priority. However, enclosure or laboratory based transmission experiments do not adequately represent the modes of transmission that may occur in the field. At present, our knowledge of the latent period relies on anecdotal observations of the time until disease emergence in a few animals that have been brought into captivity. A systematic program to bring animals from diseased areas into captivity and then to record the time until disease emergence would at least provide lower bounds on the latent period. Given information on the force of infection in the areas from which the animals were captured, it should be possible to obtain an estimate of the latent period. Some methods that have been developed to estimate incubation or latent periods in human diseases such as SARS (Kuk and Ma, 2005) or AIDS (Ronald, 2001) may be applicable.
A critical question is the value of a passive monitoring program, such as we have been applying to date. The trapping program is expensive, consuming approximately $300,000 annually. Passive monitoring has been described as:
“an inefficient use of scarce conservation funding …a form of political and intellectual displacement behavior …or worse, a deliberate delaying tactic”(Nichols and Williams, 2006).
Spotlighting surveys, carried out with the specific aim of monitoring the impact of management practices on native browsing mammal species, in which other mammal species (such as devils) were incidentally monitored, were crucial in demonstrating a significant DFTD-associated decline in the overall devil population (Hawkins et al., 2006). The resource-intensive trapping program has provided at least five key benefits. First, the monitoring was essential to determining the extent of the problem. In the absence of this systematic program, it would not have been evident that the disease was invariably fatal in the field, nor that it was spreading spatially and behaved within host populations as an infectious disease. Second, the program has shown that the disease persists within affected populations and does not disappear once populations have been sufficiently reduced. These two conclusions are essential in justifying the need for aggressive (and expensive) management interventions. Third, passive monitoring of the progression of epidemics within unmanipulated populations should enable us to estimate epidemiological parameters that can be used to build models to assist in the management of the disease. In particular, estimates of the basic reproductive rate R 0 are required to determine whether or not removal of infected animals is likely to be a successful control strategy. Fourth, control of any invasive organism is best focused at the edges of the invasion front. Monitoring is necessary to identify where the front might be. Finally, information on the progression of the disease within unmanipulated populations can be used to compare with disease progression in manipulated populations (this issue) to assess whether the disease control program is effective.
For the future, our data on distribution, rate of spread, and rate of overall population decline continue to be critical to maintaining support and to management planning, especially in decisions on the timing and location of collection of individuals for insurance populations to be isolated from the disease. At our current state of knowledge of DFTD, these estimates may change significantly and so ongoing monitoring is required. At this stage, we therefore view ongoing research into DFTD outside management trials as an essential, additional tool in the rapid identification of the best disease management strategy.
References
de Castro F, Bolker B (2005) Mechanisms of disease-induced extinction. Ecology Letters 8:117–126
Gog J, Woodroffe R, Swinton J (2002) Disease in endangered metapopulations: the importance of alternative hosts. Proceedings of the Royal Society of London. Series B: Biological Sciences (London) 269:671–676
Grenfell BT, Anderson RM (1985) The estimation of age-related rates of infection from case notifications and serological data. Journal of Hygiene 95:419–436
Guiler ER (1970) Observations on the Tasmanian devil, Sarcophilus harrisii (Marsupialia:Dasyuridae) I. Numbers, home range, movements, and food in two populations. Australian Journal of Zoology 18:49–62
Hamede R, Jones ME, McCallum H (2007) Seasonal, demographic and density-related patterns of contact between Tasmanian devils (Sarcophilus harrisii): Implications for transmission of devil facial tumour disease. Austral Ecology (in press)
Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, Lazenby B, et al. (2006) Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 13:307–324
Kuk AYC, Ma S (2005) The estimation of SARS incubation distribution from serial interval data using a convolution likelihood. Statistics in Medicine 24:2525–2537
Lachish S, Jones ME, McCallum HI (2007) The impact of devil facial tumour disease on the survival and population growth rate of the Tasmanian devil. Journal of Animal Ecology 76:926–936
Loh R, Bergfeld J, Hayes D, O’Hara A, Pyecroft S, Raidal S, et al. (2006) The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). Veterinary Pathology 43:890–895
May RM, Anderson RM (1978) Regulation and stability of host-parasite interactions. II. Destabilizing processes. Journal of Animal Ecology 47:249–267
McCallum HI, Dobson AP (1995) Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology and Evolution 10:190–194
McCallum H, Dobson A (2002) Disease, habitat fragmentation and conservation. Proceedings of the Royal Society of London. Series B: Biological Sciences (London) 269:2041–2049
McCallum H, Barlow ND, Hone J (2001) How should transmission be modelled? Trends in Ecology and Evolution 16:295–300
Nichols JD, Hines JE (2002) Approaches for the direct estimation of lambda, and demographic contributions to lambda, using capture-recapture data. Journal of Applied Statistics 29:539–568
Nichols JD, Williams BK (2006) Monitoring for conservation. Trends in Ecology & Evolution 21:668–673
Pemberton D (1990) Social organisation and behaviour of the Tasmanian devil, Sarcophilus harrisii. Ph.D. thesis, University of Tasmania
Pradel R (1996) Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 52:703–709
Ronald BG (2001) Methods for estimating the AIDS incubation time distribution when date of seroconversion is censored. Statistics in Medicine 20:795–812
Acknowledgments
H.M., M.J., and S.L. are partially funded under an Australian Research Council Linkage Grant. We are grateful to a large number of volunteers who have made this work possible. All fieldwork was carried out under animal ethics approvals to the University of Tasmania, The University of Queensland, and the Tasmanian Department of Primary Industries and Water.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCallum, H., Tompkins, D.M., Jones, M. et al. Distribution and Impacts of Tasmanian Devil Facial Tumor Disease. EcoHealth 4, 318–325 (2007). https://doi.org/10.1007/s10393-007-0118-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-007-0118-0