Abstract
Aim
This study aimed to examine the prognostic value of desmoplastic reaction (DR) in esophageal squamous cell carcinoma (ESCC), particularly in patients who received neoadjuvant therapy, such as chemotherapy (NAC) or chemoradiotherapy (NACRT).
Method
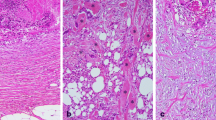
In total, 153 patients with pStage II/III ESCC were included in this study. Ninety-one patients received neoadjuvant therapy (NAC, 70; NACRT, 21). Patients were classified according to three DR categories based on the presence of keloid-like collagen and/or myxoid stroma.
Results
In total, 50, 50, and 53 patients were classified as having mature, intermediate, and immature DR, respectively. The weighted kappa coefficient was 0.623 in the patients with preoperative treatments and 0.782, in those without. The 5-year disease-specific survival (DSS) rates in patients with intermediate/immature DR was significantly worse than those with mature DR (40.7% vs. 73.3%, p < 0.001). Similarly, the 5-year DSS rate in patients with intermediate/immature DR was significantly worse than those with mature DR in a study of patients who received neoadjuvant therapy (46.7% vs. 71.2%, p = 0.009). Multivariate analysis revealed that DR (hazard ratio [HR]: 3.15, 95% confidence interval [CI] 1.58–6.27, p = 0.001), along with N factors, was an independent risk factor for DSS. Moreover, multivariate analysis of patients who received neoadjuvant therapy revealed only DR (HR: 2.47, 95% CI 1.02–5.96, p = 0.045) as independent risk factors for DSS.
Conclusion
The DR classification was a valuable prognostic factor not only in the ESCC patients without neoadjuvant therapy but also in those with neoadjuvant therapy.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Batra R, Malhotra GK, Singh S, et al. Managing squamous cell esophageal cancer. Surg Clin N Am. 2019;99:529–41.
Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of locally advanced esophageal carcinoma: ASCO Guideline: ASCO Guideline. J Clin Oncol. 2020;38:2677–94.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43.
Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–203.
Ueno H, Jones A, Jass JR, et al. Clinicopathological significance of the “keloid-like” collagen and myxoid stroma in advanced rectal cancer. Histopathology. 2002;40:327–34.
Ueno H, Kanemitsu Y, Sekine S, et al. Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol. 2017;41:1506–12.
Ueno H, Sekine S, Oshiro T, et al. Disentangling the prognostic heterogeneity of stage III colorectal cancer through histologic stromal categorization. Surgery. 2018;163:777–83.
Ueno H, Ishiguro M, Nakatani E, et al. Prognostic value of desmoplastic reaction characterisation in stage II colon cancer: prospective validation in a Phase 3 study (SACURA Trial). Br J Cancer. 2021;124:1088–97.
Wang LM, Silva MA, D’Costa Z, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183–94.
Kojima S, Hisaka T, Midorikawa R, et al. Prognostic impact of desmoplastic reaction evaluation for intrahepatic cholangiocarcinoma. Anticancer Res. 2020;40:4749–54.
Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–7.
Sakai A, Nakashima Y, Miyashita Y, et al. Histological categorisation of the desmoplastic reaction is a predictor of patient prognosis in oesophageal squamous cell carcinoma. Histopathology. 2021;79:219–26.
Li ZW, He L, Zheng Z, et al. Combined assessment of tumour cell nest size and desmoplastic reaction as an excellent prognostic predictor in oesophageal squamous cell carcinoma. Histopathology. 2022;80:1112–20.
Brierley JD, Gospodarowicz MK, Christian W. TNM Classification of Malignant Tumours. 8th ed. New York: Wiley; 2017.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1–36.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37–65.
Ueno H, Jones AM, Wilkinson KH, et al. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53:581–6.
Ueno H, Kajiwara Y, Ajioka Y, et al. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Jpn J Clin Oncol. 2021;51:1004–12.
Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–8.
Ueno H, Shinto E, Hashiguchi Y, et al. In rectal cancer, the type of desmoplastic response after preoperative chemoradiotherapy is associated with prognosis. Virchows Arch. 2015;466:655–63.
Sinn M, Denkert C, Striefler JK, et al. α-smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer. 2014;111:1917–23.
Kouzu K, Nearchou IP, Kajiwara Y, et al. Deep-learning-based classification of desmoplastic reaction on H&E predicts poor prognosis in oesophageal squamous cell carcinoma. Esophagus. 2022;81:255–63.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Hashimoto M, Uesugi N, Sugai M, et al. Desmoplastic reactions and epithelial-mesenchymal transition proteins in stages II and III colorectal cancer: association with and prognostic value for disease-free survival. Virchows Arch. 2022;480:793–805.
Ueno H, Shinto E, Shimazaki H, et al. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22:1504–12.
Ao T, Mochizuki S, Kajiwara Y, et al. Cancer-associated fibroblasts at the unfavorable desmoplastic stroma promote colorectal cancer aggressiveness: potential role of ADAM9. Int J Cancer. 2022;150:1706–21.
Tsutsumi S, Saeki H, Nakashima Y, et al. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017;108:1119–27.
Chen L, Xiong Y, Li J, et al. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell Physiol Biochem. 2017;42:2267–80.
Liu X, He M, Li L, et al. EMT and cancer cell stemness associated With chemotherapeutic resistance in esophageal cancer. Front Oncol. 2021;11: 672222.
Kong D, Long D, Liu B, et al. Downregulation of long non-coding RNA LOC101928477 correlates with tumor progression by regulating the epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Thorac Cancer. 2021;12:1303–11.
Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48.
Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6:128.
Author information
Authors and Affiliations
Contributions
Conceptualization, KK, YK and HU; writing—original draft preparation, KK and YK; writing—review and editing, HT, SM, KO, ES, YK, and SM; project administration, KK, YK and HU. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Statement
All procedures followed were in accordance with the Helsinki Declaration of 1964 and later versions. The study protocol was approved by the Institutional Review Board of the National Defense Medical College (3125). Written informed consent was obtained from all participants in the study.
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10388_2023_996_MOESM3_ESM.pptx
Supplementary Fig. 2 Disease-specific survival in esophageal squamous cell carcinoma patients differentiated using 3-tiers desmoplastic reaction classification. There is a significant difference in prognosis between mature and intermediate, and between mature and immature. In contrast, there is no significant difference in prognosis between intermediate and immature (PPTX 69 KB)
10388_2023_996_MOESM4_ESM.pptx
Supplementary Fig. 3 The cumulative recurrence rate after surgery. In patients with intermediate/immature, the cumulative recurrence rate was significantly higher than in those with mature DR (p = 0.023) (PPTX 71 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kouzu, K., Kajiwara, Y., Tsujimoto, H. et al. Prognostic impact of desmoplastic reaction in esophageal squamous cell carcinoma patients with neoadjuvant therapy. Esophagus 20, 474–483 (2023). https://doi.org/10.1007/s10388-023-00996-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-023-00996-z