Abstract
Background
We determined the serum concentrations of Programmed cell death-1 (PD-1) and its ligands (PD-L1 and PD-L2) in patients with esophageal squamous cell carcinoma (ESCC).
Methods
Blood samples were collected from 85 patients with histologically proved ESCC. Serum levels of PD-1, PD-L1, and PD-L2 were measured using enzyme linked immunosorbent assays. Correlations between serum PD-1, PD-L1, and PD-L2 concentration and tumor depth, number of lymph node metastases, organ metastasis status, or disease stage were assessed and five-year survival rates according to clinicopathological characteristics were calculated.
Results
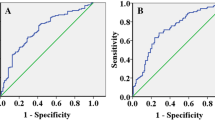
The concentration of PD-1 was not differed according to tumor progression. On the other hand, the average concentration of PD-L1 in patients with T3/T4 disease was 15.6 (12.2–18.3) pg/mL (25–75%), and this was significantly higher than that in patients with Tis/T1/T2 disease (p = 0.020). Similarly, PD-L1 levels were significantly higher in patients with positive lymph nodes than in cases with negative lymph node involvement (p = 0.006) and were higher in patients with organ metastasis (p = 0.123) and in more advanced stage (p = 0.006). Similar tendency was observed regarding PD-L2 concentrations. PD-L2 concentration was higher in T3, T4 cases (p = 0.008), in LN positive cases (p = 0.032), and in more advanced stage (p = 0.024).
Conclusion
Our data showed that a concentration of PD-L1 in peripheral blood was high in advanced cancer and high concentration of PD-L1 predicted disease progression and also poor survival in patients with ESCC.

Similar content being viewed by others
References
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2008. Esophagus. 2015;12(2):130–57.
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2007. Esophagus. 2015;12(2):101–29.
Akutsu Y, Uesato M, Shuto K, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. Ann Surg. 2013;257(6):1032–8.
Holscher AH, Bollschweiler E, Schroder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254(5):802–7 (discussion 7–8).
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95.
Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88(1):24–33.
Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–9.
Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53.
Chen Y, Wang Q, Shi B, et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56(2):231–8.
Chen L, Deng H, Lu M, et al. B7-H1 expression associates with tumor invasion and predicts patient’s survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7(9):6015–23.
Loos M, Langer R, Schuster T, et al. Clinical significance of the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac Surg. 2011;91(4):1025–31.
Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 expression marks Barrett’s esophagus and esophageal adenocarcinoma. Cancer Immunol Res. 2015;3(10):1123–9.
Qiu H, Zheng L, Tang W, Yin P, Cheng F, Wang L. Programmed death-1 (PD-1) polymorphisms in Chinese patients with esophageal cancer. Clin Biochem. 2014;47(7–8):612–7.
Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10(6):e0131403.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7.
Terawaki S, Chikuma S, Shibayama S, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186(5):2772–9.
Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol Sci. 2015;36(9):587–95.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (C) (Grant no. 15K06867) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
The participants in this study provided informed consent, and the study design was approved by the appropriate ethics review boards.
Conflict of interest
The authors declare that there are no conflicts of interest to declare. This manuscript has been checked by an English language editing service.
Rights and permissions
About this article
Cite this article
Akutsu, Y., Murakami, K., Kano, M. et al. The concentration of programmed cell death-ligand 1 in the peripheral blood is a useful biomarker for esophageal squamous cell carcinoma. Esophagus 15, 103–108 (2018). https://doi.org/10.1007/s10388-018-0604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-018-0604-1