Abstract
Purpose
In Japan, chemoradiotherapy (CRT) is primarily indicated for T4 esophageal cancer for curative intent or when aiming for downstaging. However, CRT yields a low rate of complete response, is associated with a high incidence of complications such as fistula, and often results in the emergence of severe lymph node metastasis or distant organ metastasis after the therapy. A safer and more effective treatment strategy is needed. The aim of this study is to evaluate the efficacy of the combination of induction chemotherapy and subsequent CRT for T4M0 esophageal squamous cell carcinoma.
Patients and methods
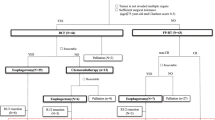
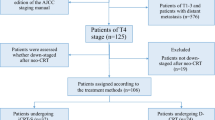
In our institute, 97 consecutive patients with T4M0 esophageal cancer underwent CRT between 2000 and 2007. Of these, 47 patients who received induction chemotherapy before CRT were eligible for the present retrospective analysis. The regimen of induction chemotherapy was FAP therapy (fluorouracil 700 mg/m2/day, cisplatin 14 mg/m2/day on days 1–5, doxorubicin 30 mg/m2/day on day 1) administered every 4 weeks. After one to five courses of FAP therapy, concurrent CRT at a dose of 60–66 Gy in 30–33 fractions was undergone.
Results
Induction chemotherapy, which preceded CRT, was effective in 21 patients (47%; responder) and ineffective in the remaining 26 patients (47%; non-responder). Better survival was achieved in the responder than in the non-responder group: the mean survival time (MST) and 1-year survival rate were 14.3 months and 66.7%, respectively, in the former, and 9.1 months and 33.6%, respectively, in the latter group. Treatment-related death occurred in 3 (6%) of the 47 patients receiving induction chemotherapy because of the progression of the radiation pneumonitis.
Conclusion
Induction chemotherapy followed by CRT is expected to improve the survival rate without increasing severe therapy-associated complications in patients with T4M0 esophageal cancer.


Similar content being viewed by others
References
Japanese Society for Esophageal Diseases. Diagnosis and Treatment Guidelines for esophageal cancer. Tokyo: Kanehara; 2007.
Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Kaneko K, Ito H, Konishi K, Kurahashi T, Ito T, Katagiri A, et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer. 2003;88:18–24.
Itoh Y, Fuwa N, Matsumoto A, Asano A, Morita K. Outcomes of radiotherapy for inoperable locally advanced (T4) esophageal cancer-retrospective analysis. Radiat Med. 2001;19:231–5.
Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, et al. Long-term toxity after definitive chemoradiotherapy for squamous cell carcinoma of thoracic esophagus. J Cin Oncol. 2003;21:2697–702.
Ajani JA, Komaki R, Putnam JB, Walsh G, Nesbitt J, Pisters PW, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer (Phila). 2001;92:279–86.
Malaisrie SC, Hofstetter WL, Correa AM, Ajani JA, Komaki RR, Rice DC, et al. The addition of induction chemotherapy to preoperative, concurrent chemoradiotherapy improves tumor response in patients with esophageal adenocarcinoma. Cancer (Phila). 2006;107:967–74.
Minsky BD, Neuberg D, Kelsen DP, Pisansky TM, Ginsberg RJ, Pajak T, et al. Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90–12): Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43:517–23.
Michel P, Adenis A, Di Fiore F, Boucher E, Galais MP, Dahan L, et al. Induction cisplatin–irinotecan followed by concurrent cisplatin–irinotecan and radiotherapy without surgery in oesophageal cancer: multicenter phase II FFCD trial. Br J Cancer. 2006;95:705–9.
Vermorken JB, Remenar E, Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Henry LR, Goldberg M, Scott W, Konski A, Meropol NJ, Freedman G, et al. Induction cisplatin and paclitaxel followed by combination chemoradiotherapy with 5-fluorouracil, cisplatin, and paclitaxel before resection in localized esophageal cancer: a phase II report. Ann Surg Oncol. 2006;13:214–20.
Honda M, Miura A, Izumi Y, Kato T, Ryotokuji T, Monma K et al. Doxorubicin, cisplatin, and fluorouracil combination therapy for metastatic esophageal squamous cell carcinoma. Dis Esophagus 2010;23:641–5.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16.
Japanese translation of common terminology criteria for adverse events (CTCAE), and instructions and guidelines. Int J Clin Oncol. 2004;9(Suppl 3):1–82.
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Radiation Therapy Oncology Group, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). JAMA. 1999;281:1623–7.
Ajani JA, Winter K, Komaki R, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551–6.
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. Clin Oncol. 2002;20:1167–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, A., Honda, M., Izumi, Y. et al. Induction chemotherapy followed by chemoradiotherapy for T4M0 esophageal cancer. Esophagus 8, 31–37 (2011). https://doi.org/10.1007/s10388-011-0255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-011-0255-y